当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequence-structure-binding relationships reveal adhesion behavior of the Car9 solid-binding peptide: an integrated experimental and simulation study
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b11617 Brittney Hellner , Sarah Alamdari , Harley Pyles , Shuai Zhang 1 , Arushi Prakash , Kayla G Sprenger , James J De Yoreo 1 , David Baker , Jim Pfaendtner , François Baneyx
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b11617 Brittney Hellner , Sarah Alamdari , Harley Pyles , Shuai Zhang 1 , Arushi Prakash , Kayla G Sprenger , James J De Yoreo 1 , David Baker , Jim Pfaendtner , François Baneyx
Affiliation
|
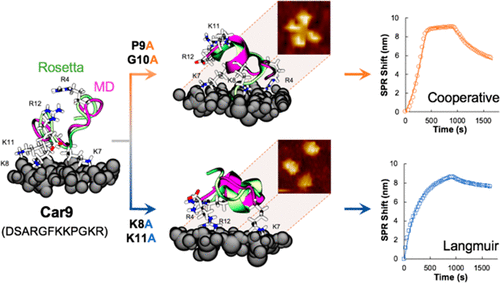
Solid-binding peptides (SBPs) recognizing inorganic and synthetic interfaces have enabled a broad range of materials science applications and hold promise as adhesive or morphogenetic control units that can be genetically encoded within desirable or designed protein frameworks. To date, the underlying relationships governing both SBP-surface and SBP-SBP interactions and how they give rise to different adsorption mechanisms remain unclear. Here, we combine protein engineering, SPR characterization, and molecular dynamics (MD) simulations initiated from Rosetta predictions to gain insights on the interplay of amino acid composition, structure, self-association and adhesion modality in a panel of variants of the Car9 silica-binding peptide (DSARGFKKPGKR) fused to the C-terminus of superfolder green fluorescent protein (sfGFP). Analysis of kinetics, energetics and MD-predicted structures shows that the high affinity binding of Car9 to the silanol-rich surface of silica is dominated by electrostatic contributions and a spectrum of several persistent interactions that, along with a high surface population of bound molecules, promote cooperative interactions between neighboring SBPs and higher order structure formation. Transition from cooperative to Langmuir adhesion in sfGFP-Car9 variants occurs in concert with a reduction of stable surface interactions and self-association, as confirmed by AFM imaging of proteins exhibiting the two different binding behaviors. We discuss the implications of these results for the de novo design of SBP-surface binding systems.
中文翻译:

序列-结构-结合关系揭示 Car9 固体结合肽的粘附行为:综合实验和模拟研究
识别无机和合成界面的固体结合肽 (SBP) 已实现广泛的材料科学应用,并有望作为粘合剂或形态发生控制单元,可以在所需或设计的蛋白质框架内进行遗传编码。迄今为止,控制 SBP-表面和 SBP-SBP 相互作用的潜在关系以及它们如何产生不同的吸附机制仍不清楚。在这里,我们结合了从 Rosetta 预测开始的蛋白质工程、SPR 表征和分子动力学 (MD) 模拟,以深入了解一组 Car9 二氧化硅变体中氨基酸组成、结构、自缔合和粘附方式的相互作用。结合肽 (DSARGFKKPGKR) 融合到超级折叠绿色荧光蛋白 (sfGFP) 的 C 端。动力学分析,能量学和 MD 预测的结构表明,Car9 与二氧化硅富含硅烷醇的表面的高亲和力结合主要由静电贡献和一系列持续相互作用的光谱决定,这些相互作用以及结合分子的高表面群体,促进了之间的协同相互作用。相邻的 SBP 和更高阶结构的形成。sfGFP-Car9 变体中从合作到朗缪尔粘附的转变与稳定表面相互作用和自缔合的减少同时发生,正如表现出两种不同结合行为的蛋白质的 AFM 成像所证实的那样。我们讨论了这些结果对 SBP 表面结合系统从头设计的影响。
更新日期:2020-01-14
中文翻译:

序列-结构-结合关系揭示 Car9 固体结合肽的粘附行为:综合实验和模拟研究
识别无机和合成界面的固体结合肽 (SBP) 已实现广泛的材料科学应用,并有望作为粘合剂或形态发生控制单元,可以在所需或设计的蛋白质框架内进行遗传编码。迄今为止,控制 SBP-表面和 SBP-SBP 相互作用的潜在关系以及它们如何产生不同的吸附机制仍不清楚。在这里,我们结合了从 Rosetta 预测开始的蛋白质工程、SPR 表征和分子动力学 (MD) 模拟,以深入了解一组 Car9 二氧化硅变体中氨基酸组成、结构、自缔合和粘附方式的相互作用。结合肽 (DSARGFKKPGKR) 融合到超级折叠绿色荧光蛋白 (sfGFP) 的 C 端。动力学分析,能量学和 MD 预测的结构表明,Car9 与二氧化硅富含硅烷醇的表面的高亲和力结合主要由静电贡献和一系列持续相互作用的光谱决定,这些相互作用以及结合分子的高表面群体,促进了之间的协同相互作用。相邻的 SBP 和更高阶结构的形成。sfGFP-Car9 变体中从合作到朗缪尔粘附的转变与稳定表面相互作用和自缔合的减少同时发生,正如表现出两种不同结合行为的蛋白质的 AFM 成像所证实的那样。我们讨论了这些结果对 SBP 表面结合系统从头设计的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号