当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Frustrated Lewis pair meditated selective single fluoride substitution in trifluoromethyl groups
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b12167 Dipendu Mandal 1 , Richa Gupta 1 , Amit K Jaiswal 1 , Rowan D Young 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b12167 Dipendu Mandal 1 , Richa Gupta 1 , Amit K Jaiswal 1 , Rowan D Young 1
Affiliation
|
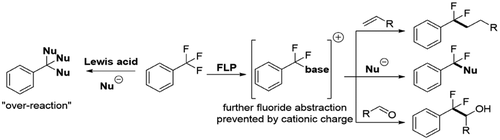
Single fluoride substitution in trifluoromethylarenes is an ongoing synthetic challenge that often leads to 'over-reaction', where multiple fluorides are replaced. Development of this reaction would allow simple access to a vast range of difluorome-thyl derivatives of current interest to pharmaceutical, agrochemistry and materials sciences. Using a Frustrated Lewis Pair approach, we have developed a generic protocol that allows a single substitution of one fluoride in trifluoromethyl groups with neutral phosphine and pyridine bases. The resulting phosphonium and pyridinium salts can be further functionalized via nucleophilic substitution, photoredox coupling and electrophilic transfer reactions allowing the generation of a vast array of difluoromethyl products.
中文翻译:

受挫的路易斯对思考三氟甲基中的选择性单氟取代
三氟甲基芳烃中的单一氟化物取代是一个持续的合成挑战,通常会导致“过度反应”,即多个氟化物被取代。该反应的发展将允许简单地获得目前对制药、农业化学和材料科学感兴趣的范围广泛的二氟甲基衍生物。使用受挫的路易斯对方法,我们开发了一个通用协议,允许用中性膦和吡啶碱单次取代三氟甲基中的一个氟化物。所得的鏻盐和吡啶盐可以通过亲核取代、光氧化还原偶联和亲电转移反应进一步官能化,从而产生大量二氟甲基产物。
更新日期:2020-01-14
中文翻译:

受挫的路易斯对思考三氟甲基中的选择性单氟取代
三氟甲基芳烃中的单一氟化物取代是一个持续的合成挑战,通常会导致“过度反应”,即多个氟化物被取代。该反应的发展将允许简单地获得目前对制药、农业化学和材料科学感兴趣的范围广泛的二氟甲基衍生物。使用受挫的路易斯对方法,我们开发了一个通用协议,允许用中性膦和吡啶碱单次取代三氟甲基中的一个氟化物。所得的鏻盐和吡啶盐可以通过亲核取代、光氧化还原偶联和亲电转移反应进一步官能化,从而产生大量二氟甲基产物。










































 京公网安备 11010802027423号
京公网安备 11010802027423号