当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyzing the Hydrodefluorination of CF3-substituted Alkenes by PhSiH3. H• Transfer from a Nickel Hydride
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b13757 Chengbo Yao 1 , Shuai Wang 1 , Jack Norton 1 , Matthew Hammond 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b13757 Chengbo Yao 1 , Shuai Wang 1 , Jack Norton 1 , Matthew Hammond 1
Affiliation
|
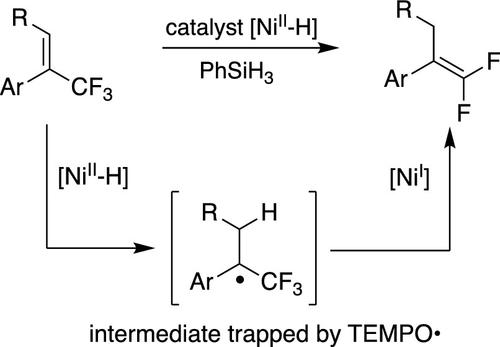
The hydrodefluorination of CF3-substituted alkenes can be catalyzed by a nickel(II) hydride bearing a pincer ligand. The cata-lyst loading can be as low as 1 mol%. gem-Difluoroalkenes containing a number of functional groups can be formed in good to excellent yields, by a radical mechanism initiated by H• transfer from the nickel hydride. The relative reactivity of various substrates supports the proposed mechanism, as does a TEMPO trapping experiment.
中文翻译:

PhSiH3催化CF3取代烯烃的加氢脱氟。H• 从镍氢化物转移
CF3 取代烯烃的加氢脱氟可以由带有钳状配体的镍 (II) 氢化物催化。催化剂负载量可以低至 1 mol%。通过从氢化镍中转移 H• 引发的自由基机制,可以以良好到极好的产率形成含有许多官能团的 gem-二氟烯烃。各种底物的相对反应性支持所提出的机制,TEMPO 捕获实验也是如此。
更新日期:2020-01-14
中文翻译:

PhSiH3催化CF3取代烯烃的加氢脱氟。H• 从镍氢化物转移
CF3 取代烯烃的加氢脱氟可以由带有钳状配体的镍 (II) 氢化物催化。催化剂负载量可以低至 1 mol%。通过从氢化镍中转移 H• 引发的自由基机制,可以以良好到极好的产率形成含有许多官能团的 gem-二氟烯烃。各种底物的相对反应性支持所提出的机制,TEMPO 捕获实验也是如此。











































 京公网安备 11010802027423号
京公网安备 11010802027423号