当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional models of nonheme diiron enzymes: reactivity of the μ-oxo-μ-1,2-peroxo-diiron(iii) intermediate in electrophilic and nucleophilic reactions.
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-01-22 , DOI: 10.1039/c9dt04551a Balázs Kripli 1 , Miklós Szávuly , Flóra Viktória Csendes , József Kaizer
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-01-22 , DOI: 10.1039/c9dt04551a Balázs Kripli 1 , Miklós Szávuly , Flóra Viktória Csendes , József Kaizer
Affiliation
|
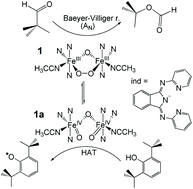
The reactivity of the previously reported peroxo-adduct [FeIII2(μ-O)(μ-1,2-O2)(IndH)2(solv)2]2+ (1) (IndH = 1,3-bis(2-pyridyl-imino)isoindoline) has been investigated in nucleophilic (e.g., deformylation of alkyl and aryl alkyl aldehydes) and electrophilic (e.g. oxidation of phenols) stoichiometric reactions as biomimics of ribonucleotide reductase (RNR-R2) and aldehyde deformylating oxygenase (ADO) enzymes. Based on detailed kinetic and mechanistic studies, we have found further evidence for the ambiphilic behaviour of the peroxo intermediates proposed for diferric oxidoreductase enzymes.
中文翻译:

非血红素二铁酶的功能模型:μ-氧代-μ-1,2-过氧二铁(iii)中间体在亲电和亲核反应中的反应性。
先前报道的过氧加合物[FeIII2(μ-O)(μ-1,2-O2)(IndH)2(solv)2] 2+(1)的反应性(IndH = 1,3-bis(2-作为核糖核苷酸还原酶(RNR-R2)和醛脱甲酰加氧酶(ADO)的仿生生物,已在亲核(例如烷基和芳基烷基醛的甲酰基化)和亲电子(例如苯酚的氧化)化学计量反应中进行了吡啶基-亚氨基)异吲哚啉研究。 。基于详细的动力学和机理研究,我们发现了提议用于二铁氧化还原酶的过氧中间体的歧义行为的进一步证据。
更新日期:2020-02-13
中文翻译:

非血红素二铁酶的功能模型:μ-氧代-μ-1,2-过氧二铁(iii)中间体在亲电和亲核反应中的反应性。
先前报道的过氧加合物[FeIII2(μ-O)(μ-1,2-O2)(IndH)2(solv)2] 2+(1)的反应性(IndH = 1,3-bis(2-作为核糖核苷酸还原酶(RNR-R2)和醛脱甲酰加氧酶(ADO)的仿生生物,已在亲核(例如烷基和芳基烷基醛的甲酰基化)和亲电子(例如苯酚的氧化)化学计量反应中进行了吡啶基-亚氨基)异吲哚啉研究。 。基于详细的动力学和机理研究,我们发现了提议用于二铁氧化还原酶的过氧中间体的歧义行为的进一步证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号