当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, photophysics and biomolecule interactive studies of new hybrid benzo-2,1,3-thiadiazoles
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020/01/15 , DOI: 10.1039/c9nj05932f José S. S. Neto 1, 2, 3, 4 , Roberta Krüger 1, 2, 3, 4 , Renata A. Balaguez 1, 2, 3, 4 , Mariana G. Fronza 2, 3, 4, 5, 6 , Thiago V. Acunha 7, 8, 9, 10, 11 , Robson S. Oliboni 2, 3, 4, 12 , Lucielli Savegnago 2, 3, 4, 5, 6 , Bernardo A. Iglesias 7, 8, 9, 10, 11 , Diego Alves 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020/01/15 , DOI: 10.1039/c9nj05932f José S. S. Neto 1, 2, 3, 4 , Roberta Krüger 1, 2, 3, 4 , Renata A. Balaguez 1, 2, 3, 4 , Mariana G. Fronza 2, 3, 4, 5, 6 , Thiago V. Acunha 7, 8, 9, 10, 11 , Robson S. Oliboni 2, 3, 4, 12 , Lucielli Savegnago 2, 3, 4, 5, 6 , Bernardo A. Iglesias 7, 8, 9, 10, 11 , Diego Alves 1, 2, 3, 4
Affiliation
|
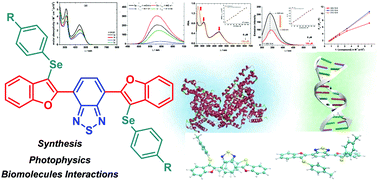
Four new hybrid molecules containing benzo-2,1,3-thiadiazole, benzofuran and arylselanyl moieties, named 4,7-bis(3-(arylselanyl)benzofuran-2-yl)benzo[c][1,2,5]thiadiazoles, were designed and synthesized in good yields through an electrophilic cyclization of 4,7-bis((2-methoxyphenyl)ethynyl)benzo[c][1,2,5]thiadiazole 1 and diaryl diselenides 2 using trichloroisocyanuric acid to generate electrophilic arylselanyl species. The photophysical properties of compounds 3a–d were investigated using UV-vis absorption and emission fluorescence analysis. The emission fluorescence analysis showed a yellow-orange region emission. Large Stokes shift values were observed for all new derivatives and attributed to the ICT state and to the electron-substituent properties. The biomolecule-binding DNA and HSA-binding interactions were also investigated by experimental and theoretical methods.
中文翻译:

新型杂合苯并-2,1,3-噻二唑的合成,光物理与生物分子相互作用的研究
四个含有苯并-2,1,3-噻二唑,苯并呋喃和芳基戊二烯基部分的新杂合分子,分别命名为4,7-双(3-(芳基硒基)苯并呋喃-2-基)苯并[ c ] [1,2,5]噻二唑通过使用三氯异氰尿酸对4,7-双((2-甲氧基苯基)乙炔基)苯并[ c ] [1,2,5]噻二唑1和二芳基二硒化物2进行亲电环化反应生成亲电的芳基硒基种类。化合物3a–d的光物理性质使用紫外可见吸收和发射荧光分析进行了研究。发射荧光分析显示橙黄色区域发射。对于所有新的导数,观察到较大的斯托克斯位移值,并且归因于ICT状态和电子取代基特性。还通过实验和理论方法研究了生物分子结合DNA和HSA结合相互作用。
更新日期:2020-02-17
中文翻译:

新型杂合苯并-2,1,3-噻二唑的合成,光物理与生物分子相互作用的研究
四个含有苯并-2,1,3-噻二唑,苯并呋喃和芳基戊二烯基部分的新杂合分子,分别命名为4,7-双(3-(芳基硒基)苯并呋喃-2-基)苯并[ c ] [1,2,5]噻二唑通过使用三氯异氰尿酸对4,7-双((2-甲氧基苯基)乙炔基)苯并[ c ] [1,2,5]噻二唑1和二芳基二硒化物2进行亲电环化反应生成亲电的芳基硒基种类。化合物3a–d的光物理性质使用紫外可见吸收和发射荧光分析进行了研究。发射荧光分析显示橙黄色区域发射。对于所有新的导数,观察到较大的斯托克斯位移值,并且归因于ICT状态和电子取代基特性。还通过实验和理论方法研究了生物分子结合DNA和HSA结合相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号