当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Correlation and Thermodynamic Analysis of Solubility of Mesotrione in Pure Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.jced.9b01063 Yujia Yang 1 , Shichao Du 1 , Weibing Dong 2 , Gang Wang 2 , Haichao Li 2 , Junbo Gong 1, 2 , Songgu Wu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.jced.9b01063 Yujia Yang 1 , Shichao Du 1 , Weibing Dong 2 , Gang Wang 2 , Haichao Li 2 , Junbo Gong 1, 2 , Songgu Wu 1
Affiliation
|
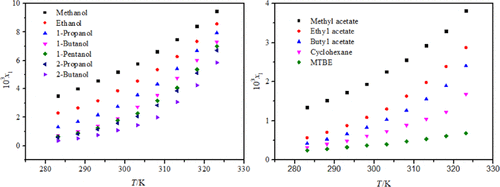
The solubility of mesotrione in 12 pure solvents was determined at a temperature ranging from 283.15 to 323.15 K using a gravimetric method. The solubility increased expectedly with temperature increasing in all solvents. Solubility in these pure solvents was well correlated by the modified Apelblat equation, λh equation, and NRTL model. The modified Apelblat model achieves the best fitting results. Based on the study of solvent properties, it was found that the polarity and hydrogen bond donor ability of solvent determine the solubility of mesotrione in alcohols, whereas in other solvents, cohesive energy density plays the key role. Finally, thermodynamic functions of mixing were derived for further understanding of the mixing process.
中文翻译:

甲基磺草酮在纯溶剂中的溶解度的相关性和热力学分析
使用重量分析法测定甲基磺草酮在12种纯溶剂中的溶解度,温度范围为283.15至323.15K。在所有溶剂中,溶解度均随着温度的升高而增加。在这些纯溶剂中的溶解度良好由修改Apelblat方程,λ相关ħ方程,NRTL模型。修改后的Apelblat模型可达到最佳拟合效果。根据对溶剂性能的研究,发现溶剂的极性和氢键供体的能力决定了甲基磺草酮在醇中的溶解度,而在其他溶剂中,内聚能密度起着关键作用。最后,推导了混合的热力学函数,以进一步理解混合过程。
更新日期:2020-01-15
中文翻译:

甲基磺草酮在纯溶剂中的溶解度的相关性和热力学分析
使用重量分析法测定甲基磺草酮在12种纯溶剂中的溶解度,温度范围为283.15至323.15K。在所有溶剂中,溶解度均随着温度的升高而增加。在这些纯溶剂中的溶解度良好由修改Apelblat方程,λ相关ħ方程,NRTL模型。修改后的Apelblat模型可达到最佳拟合效果。根据对溶剂性能的研究,发现溶剂的极性和氢键供体的能力决定了甲基磺草酮在醇中的溶解度,而在其他溶剂中,内聚能密度起着关键作用。最后,推导了混合的热力学函数,以进一步理解混合过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号