当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Physical Characterization of Frozen Aqueous Solutions Containing Sodium Chloride and Humic Acid at Environmentally Relevant Temperatures
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-01-24 , DOI: 10.1021/acsearthspacechem.9b00319 Subha Chakraborty 1, 2 , Tara F. Kahan 1, 2
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-01-24 , DOI: 10.1021/acsearthspacechem.9b00319 Subha Chakraborty 1, 2 , Tara F. Kahan 1, 2
Affiliation
|
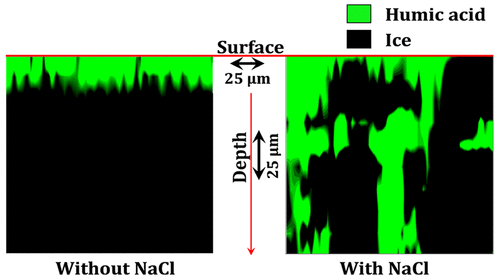
Sea ice presents a complex reaction medium containing poorly understood distributions of solutes, ice, and sometimes liquid brine. We studied the three-dimensional distribution of liquid brine and dissolved organic matter using confocal Raman microscopy at environmentally relevant sodium chloride (NaCl) and dissolved carbon concentrations in frozen ternary solutions containing NaCl and humic acid (HA). While HA is predominantly excluded to the ice surface in the absence of salt, it coexists with liquid brine in the micrometer scale channels throughout the entire ice sample when salt is present. HA increased the extent of liquid coverage of the ice surface at each temperature studied, but the total amount of liquid water at the surface did not increase. This suggests that HA promotes spreading of liquid water at the surface of frozen salt solutions, but does not increase the extent of surface melting. HA suppressed the phase transition from solid ice + liquid brine to solid ice + solid hydrohalite (NaCl·2H2O) when samples were cooled. Phase-transition temperatures ranged from (−26.4 ± 0.6) °C (with 0.003 mg/L HA) to (−33.7 ± 0.5) °C (with 30 mg/L HA), compared to (−22.3 ± 0.5) °C in the absence of HA. The phase transition in the reverse direction (as the samples were warmed) occurred at (−21.8 ± 0.5) °C, very close to the Eutectic temperature (−21.1 °C) at all HA concentrations and in the absence of HA.
中文翻译:

在环境相关温度下含有氯化钠和腐殖酸的冷冻水溶液的物理特性
海冰呈现出复杂的反应介质,其中包含对溶质,冰以及有时为液态盐水的分布知之甚少。我们使用共聚焦拉曼显微镜在与环境相关的氯化钠(NaCl)和含NaCl和腐殖酸(HA)的冷冻三元溶液中研究了溶解的碳浓度,使用共聚焦拉曼显微镜研究了液态盐水和溶解的有机物的三维分布。尽管在没有盐的情况下,主要将HA排除在冰表面,但当存在盐时,它会在整个冰样品的微米级通道中与液态盐水共存。HA在每个研究温度下都增加了冰表面的液体覆盖程度,但表面的液态水总量并未增加。这表明HA促进了液态水在冷冻盐溶液表面的扩散,但不会增加表面熔化的程度。HA抑制了从固态冰+液态盐水到固态冰+固态氢卤石(NaCl·2H2 O)冷却样品。相变温度范围为(−26.4±0.6)°C(使用0.003 mg / L HA)至(−33.7±0.5)°C(使用30 mg / L HA),而(−22.3±0.5)°C在没有医管局的情况下。反向(随着样品变热)的相变发生在(−21.8±0.5)°C,在所有HA浓度下和没有HA的情况下都非常接近共晶温度(−21.1°C)。
更新日期:2020-01-26
中文翻译:

在环境相关温度下含有氯化钠和腐殖酸的冷冻水溶液的物理特性
海冰呈现出复杂的反应介质,其中包含对溶质,冰以及有时为液态盐水的分布知之甚少。我们使用共聚焦拉曼显微镜在与环境相关的氯化钠(NaCl)和含NaCl和腐殖酸(HA)的冷冻三元溶液中研究了溶解的碳浓度,使用共聚焦拉曼显微镜研究了液态盐水和溶解的有机物的三维分布。尽管在没有盐的情况下,主要将HA排除在冰表面,但当存在盐时,它会在整个冰样品的微米级通道中与液态盐水共存。HA在每个研究温度下都增加了冰表面的液体覆盖程度,但表面的液态水总量并未增加。这表明HA促进了液态水在冷冻盐溶液表面的扩散,但不会增加表面熔化的程度。HA抑制了从固态冰+液态盐水到固态冰+固态氢卤石(NaCl·2H2 O)冷却样品。相变温度范围为(−26.4±0.6)°C(使用0.003 mg / L HA)至(−33.7±0.5)°C(使用30 mg / L HA),而(−22.3±0.5)°C在没有医管局的情况下。反向(随着样品变热)的相变发生在(−21.8±0.5)°C,在所有HA浓度下和没有HA的情况下都非常接近共晶温度(−21.1°C)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号