当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Middle-Down Proteomic Analyses with Ion Mobility Separations of Endogenous Isomeric Proteoforms.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.analchem.9b05011 Pavel V Shliaha 1 , Vladimir Gorshkov 1 , Sergey I Kovalchuk 1 , Veit Schwämmle 1 , Matthew A Baird 2 , Alexandre A Shvartsburg 2 , Ole N Jensen 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.analchem.9b05011 Pavel V Shliaha 1 , Vladimir Gorshkov 1 , Sergey I Kovalchuk 1 , Veit Schwämmle 1 , Matthew A Baird 2 , Alexandre A Shvartsburg 2 , Ole N Jensen 1
Affiliation
|
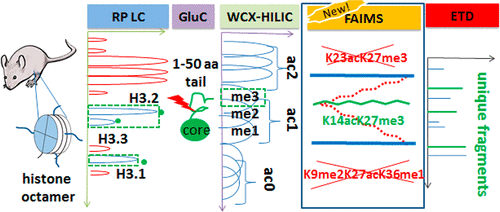
Biological functions of many proteins are governed by post-translational modifications (PTMs). In particular, the rich PTM complement in histones controls the gene expression and chromatin structure with major health implications via a combinatoric language. Deciphering that "histone code" is the great challenge for proteomics given an astounding number of possible proteoforms, including isomers with different PTM positions. These must be disentangled on the top- or middle-down level to preserve the key PTM connectivity, which condensed-phase separations failed to achieve. We reported the capability of ion mobility spectrometry (IMS) methods to resolve such isomers for model histone tails. Here, we advance to biological samples, showing middle-down analyses of histones from mouse embryonic stem cells via online chromatography to fractionate proteoforms with distinct PTM sets, differential or field asymmetric waveform IMS (FAIMS) to resolve the isomers, and Orbitrap mass spectrometry with electron transfer dissociation to identify the resolved species.
中文翻译:

内源性异构体蛋白形式的离子迁移分离的中下蛋白质组学分析。
许多蛋白质的生物学功能受翻译后修饰(PTM)的控制。特别是,组蛋白中丰富的PTM补体通过组合语言控制着对健康有重大影响的基因表达和染色质结构。考虑到惊人的可能的蛋白质形式,包括具有不同PTM位置的异构体,对蛋白质组学来说,“组蛋白密码”的理解是一个巨大的挑战。必须在上层或中下层解开这些关系,以保持关键的PTM连接性,而冷凝相分离则无法实现。我们报告了离子迁移谱(IMS)方法解决模型组蛋白尾巴这种异构体的能力。在这里,我们进入生物样本,
更新日期:2020-01-21
中文翻译:

内源性异构体蛋白形式的离子迁移分离的中下蛋白质组学分析。
许多蛋白质的生物学功能受翻译后修饰(PTM)的控制。特别是,组蛋白中丰富的PTM补体通过组合语言控制着对健康有重大影响的基因表达和染色质结构。考虑到惊人的可能的蛋白质形式,包括具有不同PTM位置的异构体,对蛋白质组学来说,“组蛋白密码”的理解是一个巨大的挑战。必须在上层或中下层解开这些关系,以保持关键的PTM连接性,而冷凝相分离则无法实现。我们报告了离子迁移谱(IMS)方法解决模型组蛋白尾巴这种异构体的能力。在这里,我们进入生物样本,











































 京公网安备 11010802027423号
京公网安备 11010802027423号