iScience ( IF 4.6 ) Pub Date : 2020-01-14 , DOI: 10.1016/j.isci.2020.100839 Cassandra A Ramos 1 , Ching Ouyang 2 , Yue Qi 3 , Yiyin Chung 3 , Chun-Ting Cheng 3 , Mark A LaBarge 4 , Victoria L Seewaldt 4 , David K Ann 1
|
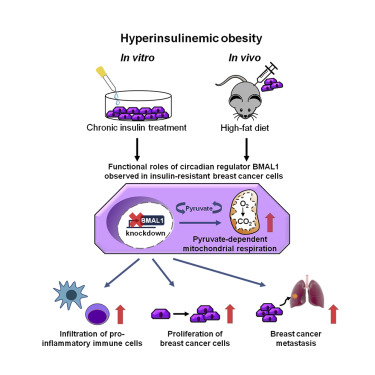
The epidemiological association between disrupted circadian rhythms and metabolic diseases is implicated in increased risk of human breast cancer and poor therapeutic outcomes. To define a metabolic phenotype and the underlying molecular mechanism, we applied chronic insulin treatment (CIT) to an in vitro model of triple-negative breast cancer to directly address how BMAL1, a key circadian transcription factor, regulates cancer cell respiration and governs tumor progression. At the cellular level, BMAL1 suppresses the flexibility of mitochondrial substrate usage and the pyruvate-dependent mitochondrial respiration induced by CIT. We established an animal model of diet-induced obesity/hyperinsulinemia and observed that BMAL1 functions as a tumor suppressor in obese, but not lean, mice. Downregulation of BMAL1 is associated with higher risk of metastasis in human breast tumors. In summary, loss of BMAL1 in tumors confers advantages to cancer cells in both intrinsic mitochondrial metabolism and extrinsic inflammatory tumor microenvironment during pre-diabetic obesity/hyperinsulinemia.
中文翻译:

BMAL1的非规范性功能限制了肥胖引起的三阴性乳腺癌。
昼夜节律紊乱与代谢疾病之间的流行病学关联牵涉到人类乳腺癌风险增加和不良治疗结果。为了定义代谢表型和潜在的分子机制,我们在体外应用了慢性胰岛素治疗(CIT)一个三阴性乳腺癌模型,可以直接解决关键的昼夜节律转录因子BMAL1如何调节癌细胞呼吸并控制肿瘤进展。在细胞水平,BMAL1抑制了线粒体底物使用的灵活性以及CIT诱导的丙酮酸依赖性线粒体呼吸。我们建立了饮食诱发的肥胖/高胰岛素血症的动物模型,并观察到BMAL1在肥胖但不瘦的小鼠中起着抑癌作用。BMAL1的下调与人类乳腺肿瘤转移的较高风险相关。总之,在糖尿病前期肥胖/高胰岛素血症期间,肿瘤中BMAL1的缺失在固有的线粒体代谢和外部炎症肿瘤微环境中都赋予癌细胞以优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号