Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cancer-Specific Loss of p53 Leads to a Modulation of Myeloid and T Cell Responses.
Cell Reports ( IF 7.5 ) Pub Date : 2020-01-14 , DOI: 10.1016/j.celrep.2019.12.028 Julianna Blagih 1 , Fabio Zani 1 , Probir Chakravarty 1 , Marc Hennequart 1 , Steven Pilley 1 , Sebastijan Hobor 1 , Andreas K Hock 2 , Josephine B Walton 3 , Jennifer P Morton 4 , Eva Gronroos 1 , Susan Mason 5 , Ming Yang 1 , Iain McNeish 6 , Charles Swanton 1 , Karen Blyth 4 , Karen H Vousden 1
Cell Reports ( IF 7.5 ) Pub Date : 2020-01-14 , DOI: 10.1016/j.celrep.2019.12.028 Julianna Blagih 1 , Fabio Zani 1 , Probir Chakravarty 1 , Marc Hennequart 1 , Steven Pilley 1 , Sebastijan Hobor 1 , Andreas K Hock 2 , Josephine B Walton 3 , Jennifer P Morton 4 , Eva Gronroos 1 , Susan Mason 5 , Ming Yang 1 , Iain McNeish 6 , Charles Swanton 1 , Karen Blyth 4 , Karen H Vousden 1
Affiliation
|
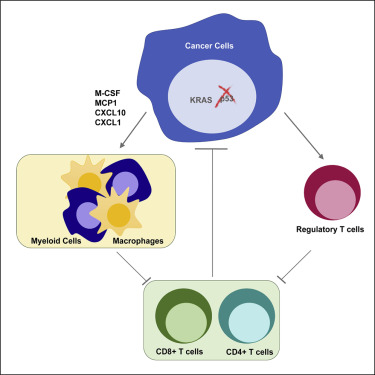
Loss of p53 function contributes to the development of many cancers. While cell-autonomous consequences of p53 mutation have been studied extensively, the role of p53 in regulating the anti-tumor immune response is still poorly understood. Here, we show that loss of p53 in cancer cells modulates the tumor-immune landscape to circumvent immune destruction. Deletion of p53 promotes the recruitment and instruction of suppressive myeloid CD11b+ cells, in part through increased expression of CXCR3/CCR2-associated chemokines and macrophage colony-stimulating factor (M-CSF), and attenuates the CD4+ T helper 1 (Th1) and CD8+ T cell responses in vivo. p53-null tumors also show an accumulation of suppressive regulatory T (Treg) cells. Finally, we show that two key drivers of tumorigenesis, activation of KRAS and deletion of p53, cooperate to promote immune tolerance.
中文翻译:

癌症特异性 p53 缺失会导致骨髓细胞和 T 细胞反应的调节。
p53 功能的丧失会导致许多癌症的发生。虽然p53突变的细胞自主后果已被广泛研究,但p53在调节抗肿瘤免疫反应中的作用仍然知之甚少。在这里,我们证明癌细胞中 p53 的缺失可以调节肿瘤免疫景观,从而避免免疫破坏。 p53 的缺失促进抑制性骨髓 CD11b+ 细胞的招募和指导,部分是通过增加 CXCR3/CCR2 相关趋化因子和巨噬细胞集落刺激因子 (M-CSF) 的表达,并减弱 CD4+ T 辅助细胞 1 (Th1) 和 CD8+ T 细胞体内反应。 p53 缺失的肿瘤还显示出抑制性调节性 T (Treg) 细胞的积累。最后,我们发现肿瘤发生的两个关键驱动因素,KRAS 的激活和 p53 的缺失,共同促进免疫耐受。
更新日期:2020-01-15
中文翻译:

癌症特异性 p53 缺失会导致骨髓细胞和 T 细胞反应的调节。
p53 功能的丧失会导致许多癌症的发生。虽然p53突变的细胞自主后果已被广泛研究,但p53在调节抗肿瘤免疫反应中的作用仍然知之甚少。在这里,我们证明癌细胞中 p53 的缺失可以调节肿瘤免疫景观,从而避免免疫破坏。 p53 的缺失促进抑制性骨髓 CD11b+ 细胞的招募和指导,部分是通过增加 CXCR3/CCR2 相关趋化因子和巨噬细胞集落刺激因子 (M-CSF) 的表达,并减弱 CD4+ T 辅助细胞 1 (Th1) 和 CD8+ T 细胞体内反应。 p53 缺失的肿瘤还显示出抑制性调节性 T (Treg) 细胞的积累。最后,我们发现肿瘤发生的两个关键驱动因素,KRAS 的激活和 p53 的缺失,共同促进免疫耐受。











































 京公网安备 11010802027423号
京公网安备 11010802027423号