当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and evaluation of pyrazole bearing α-aminophosphonate derivatives as potential acetylcholinesterase inhibitors against Alzheimer's disease.
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.bioorg.2020.103589 Sarfaraz Shaikh 1 , Pratik Dhavan 2 , Ganesh Pavale 1 , M M V Ramana 1 , B L Jadhav 2
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.bioorg.2020.103589 Sarfaraz Shaikh 1 , Pratik Dhavan 2 , Ganesh Pavale 1 , M M V Ramana 1 , B L Jadhav 2
Affiliation
|
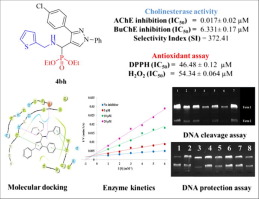
A series of novel scaffold of N-substituted pyrazole derived α-aminophosphonates were designed, synthesized and evaluated for their anti-cholinesterase activity. Porcine pancreatic lipase (PPL) was used as a catalyst for the organic transformation. Compounds 4ah and 4bh proved to be more potent than the standard drug tacrine, rivastigmine and galantamine for AChE inhibition activity with IC50 value between 0.055 ± 0.143 µM and 0.017 ± 0.02 µM respectively. BuChE activity of the synthesized derivatives possessed moderate to weak inhibition potency. 4bhshows a comparable activity to Rivastigmine against BuChE (IC50 = 6.331 ± 0.17). The compounds did not show any cytotoxicity against HEK-293 cells when compared to standard drugs. Cell viability assay using N2a cell showed compounds 4ah and 4bh showed comparable results to positive control rivastigmine. In addition, these compounds showed promising antioxidant activities against DPPH and H2O2 scavenging. Both 4ah and 4bh showed mixed-type inhibition which supported by molecular docking studies by acting as a dual site inhibitor. The predicted ADME showed good pharmacokinetics as predicted by QikProp. DNA cleavage studies and DNA protection assay of active compounds were also performed. 4bh did not show any damage to DNA and was protective in nature.
中文翻译:

设计,合成和评估带有α-氨基膦酸酯衍生物的吡唑,作为对抗阿尔茨海默氏病的潜在乙酰胆碱酯酶抑制剂。
设计,合成和评估了一系列新型的N-取代的吡唑衍生的α-氨基膦酸酯支架,并评估了它们的抗胆碱酯酶活性。猪胰脂肪酶(PPL)用作有机转化的催化剂。事实证明,化合物4ah和4bh对AChE抑制活性比标准药物他克林,卡巴拉汀和加兰他敏更有效,IC50值分别在0.055±0.143 µM和0.017±0.02 µM之间。合成衍生物的BuChE活性具有中等至弱的抑制能力。4bh显示出与Rivastigmine对抗BuChE相当的活性(IC50 = 6.331±0.17)。与标准药物相比,该化合物对HEK-293细胞没有任何细胞毒性。使用N2a细胞进行的细胞活力测定显示,化合物4ah和4bh与阳性对照卡巴拉汀相比具有可比的结果。此外,这些化合物显示出抗DPPH和H2O2清除的抗氧化剂活性。4ah和4bh均显示出混合型抑制作用,这通过分子对接研究作为双位点抑制剂得到了支持。如QikProp所预测,预测的ADME显示出良好的药代动力学。还进行了活性化合物的DNA裂解研究和DNA保护分析。4bh不会对DNA造成任何损害,并且具有保护性。如QikProp所预测,预测的ADME显示出良好的药代动力学。还进行了活性化合物的DNA裂解研究和DNA保护分析。4bh不会对DNA造成任何损害,并且具有保护性。如QikProp所预测,预测的ADME显示出良好的药代动力学。还进行了活性化合物的DNA裂解研究和DNA保护分析。4bh不会对DNA造成任何损害,并且具有保护性。
更新日期:2020-01-15
中文翻译:

设计,合成和评估带有α-氨基膦酸酯衍生物的吡唑,作为对抗阿尔茨海默氏病的潜在乙酰胆碱酯酶抑制剂。
设计,合成和评估了一系列新型的N-取代的吡唑衍生的α-氨基膦酸酯支架,并评估了它们的抗胆碱酯酶活性。猪胰脂肪酶(PPL)用作有机转化的催化剂。事实证明,化合物4ah和4bh对AChE抑制活性比标准药物他克林,卡巴拉汀和加兰他敏更有效,IC50值分别在0.055±0.143 µM和0.017±0.02 µM之间。合成衍生物的BuChE活性具有中等至弱的抑制能力。4bh显示出与Rivastigmine对抗BuChE相当的活性(IC50 = 6.331±0.17)。与标准药物相比,该化合物对HEK-293细胞没有任何细胞毒性。使用N2a细胞进行的细胞活力测定显示,化合物4ah和4bh与阳性对照卡巴拉汀相比具有可比的结果。此外,这些化合物显示出抗DPPH和H2O2清除的抗氧化剂活性。4ah和4bh均显示出混合型抑制作用,这通过分子对接研究作为双位点抑制剂得到了支持。如QikProp所预测,预测的ADME显示出良好的药代动力学。还进行了活性化合物的DNA裂解研究和DNA保护分析。4bh不会对DNA造成任何损害,并且具有保护性。如QikProp所预测,预测的ADME显示出良好的药代动力学。还进行了活性化合物的DNA裂解研究和DNA保护分析。4bh不会对DNA造成任何损害,并且具有保护性。如QikProp所预测,预测的ADME显示出良好的药代动力学。还进行了活性化合物的DNA裂解研究和DNA保护分析。4bh不会对DNA造成任何损害,并且具有保护性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号