Water Research ( IF 11.4 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.watres.2020.115504 Zhen Wang , Wei Qiu , Suyan Pang , Yuan Gao , Yang Zhou , Ying Cao , Jin Jiang
|
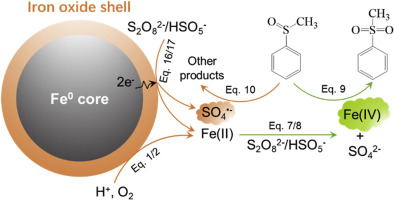
Activation of persulfates (i.e., peroxydisulfate (PDS) and peroxymonosulfate (PMS)) by nanoscale zero-valent iron (nZVI) is reported to be effective in oxidative treatment of environmental contaminants. It has been widely accepted in numerous literature that sulfate radical (SO4•-) formed from the decomposition of persulfates activated by aqueous Fe(II) released from nZVI corrosion is responsible for the oxidative performance in nZVI/persulfates systems. In this work, by employing methyl phenyl sulfoxide (PMSO) as a probe, we demonstrated that the activation of persulfates by nZVI through electron transfer led to SO4•- formation, while the homogeneous activation of persulfate by the released Fe(II) resulted in ferryl ion species (Fe(IV)) generation in nZVI/persulfates systems. Similarly, nanoscale zero-valent aluminum (nZVAl) and zinc (nZVZn) were also demonstrated to be able to donate electron to persulfates leading to SO4•- formation. However, the insulative aluminum oxide shell hindered the electron transfer leading to the poor persulfates decomposition, while the conductive iron and zinc oxide shell enabled the electron transfer process resulting in a continuous generation of SO4•-. Further, it was obtained that the relative contribution of SO4•- and Fe(IV) in nZVI/persulfates systems was independent of the initial concentration of nZVI and PDS, but was positively correlated with PMS concentration. In addition, the increases of pH from 3 to 7 led to the decrease of the relative contribution of Fe(IV), which was rationalized by the decrease of availability of aqueous Fe(II) at higher pH. Our findings not only shed lights on the nature of the reactive intermediate formed in the nZVI/persulfates systems, but also unprecedentedly distinguished the surface activation of persulfates from the homogeneous catalysis process.
中文翻译:

纳米零价铁活化过氧二硫酸盐和过氧一硫酸盐过程中形成的亚硫酸根离子种类(Fe(IV))和硫酸根的相对贡献
据报道,纳米级零价铁(nZVI)活化过硫酸盐(即过氧二硫酸盐(PDS)和过氧单硫酸盐(PMS))在氧化处理环境污染物方面是有效的。在许多文献中已被广泛接受,由nZVI腐蚀释放的Fe(II)水溶液活化过硫酸盐分解而形成的硫酸根(SO 4 •-)是nZVI /过硫酸盐系统中氧化性能的原因。在这项工作中,通过使用甲基苯基亚砜(PMSO)作为探针,我们证明了nZVI通过电子转移激活过硫酸盐导致SO 4 •-形成,而过硫酸盐被释放的Fe(II)均匀激活导致nZVI /过硫酸盐系统中生成Ferryl离子物种(Fe(IV))。同样,纳米级零价铝(nZVAl)和锌(nZVZn)也被证明能够将电子捐赠给过硫酸盐,从而形成SO 4 •。但是,绝缘氧化铝壳阻碍了电子转移,导致过硫酸盐分解不充分,而导电铁和氧化锌壳使电子转移过程得以实现,从而连续产生SO 4 •-。此外,获得了SO 4 •-的相对贡献。nZVI /过硫酸盐系统中的Fe(IV)与nZVI和PDS的初始浓度无关,但与PMS浓度呈正相关。此外,pH从3增加到7导致Fe(IV)的相对贡献降低,这可以通过在较高pH下降低Fe(II)水溶液的有效性来实现。我们的发现不仅揭示了nZVI /过硫酸盐体系中形成的反应性中间体的性质,而且空前地区分了均相催化过程中过硫酸盐的表面活化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号