Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Binding Recognition and Susceptibility of Tofacitinib toward Janus Kinases.
ACS Omega ( IF 3.7 ) Pub Date : 2020-01-02 , DOI: 10.1021/acsomega.9b02800 Kamonpan Sanachai 1 , Panupong Mahalapbutr 1 , Kiattawee Choowongkomon 2 , Rungtiva P Poo-Arporn 3 , Peter Wolschann 4, 4 , Thanyada Rungrotmongkol 1, 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-01-02 , DOI: 10.1021/acsomega.9b02800 Kamonpan Sanachai 1 , Panupong Mahalapbutr 1 , Kiattawee Choowongkomon 2 , Rungtiva P Poo-Arporn 3 , Peter Wolschann 4, 4 , Thanyada Rungrotmongkol 1, 1
Affiliation
|
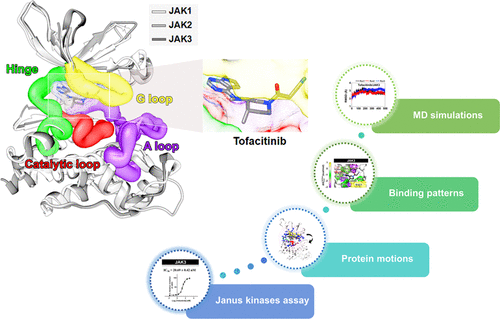
Janus kinases (JAKs) are enzymes involved in signaling pathways that affect hematopoiesis and immune cell functions. JAK1, JAK2, and JAK3 play different roles in numerous diseases of the immune system and have also been considered as potential targets for cancer therapy. In the present study, the susceptibility of the oral JAK inhibitor tofacitinib against these three JAKs was elucidated using the 500-ns molecular dynamics (MD) simulations and free energy calculations based on MM-PB(GB)SA, QM/MM-GBSA (PM3 and SCC-DFTB), and SIE methods. The obtained results revealed that tofacitinib could interact with all JAKs at the ATP-binding site via electrostatic attraction, hydrogen bond formation, and in particular van der Waals interaction. The conserved glutamate and leucine residues (E957 and L959 of JAK1, E930 and L932 of JAK2, and E903 and L905 of JAK3) located in the hinge region stabilized tofacitinib binding through strongly formed hydrogen bonds. Complexation with the incoming tofacitinib led to a closed conformation of the ATP-binding site and a decreased protein fluctuation at the glycine loop of the JAK protein. The binding affinities of tofacitinib/JAKs were ranked in the order of JAK3 > JAK2 ∼ JAK1, which are in line with the reported experimental data.
中文翻译:

深入了解Tofacitinib对Janus激酶的结合识别和敏感性。
Janus激酶(JAKs)是参与影响造血和免疫细胞功能的信号通路的酶。JAK1,JAK2和JAK3在多种免疫系统疾病中扮演不同的角色,也被认为是癌症治疗的潜在靶标。在本研究中,使用500 ns分子动力学(MD)模拟和基于MM-PB(GB)SA,QM / MM-GBSA的自由能计算,阐明了口服JAK抑制剂tofacitinib对这三种JAK的敏感性( PM3和SCC-DFTB)以及SIE方法。获得的结果表明托法替尼可以通过静电吸引,氢键形成,特别是范德华相互作用与ATP结合位点上的所有JAK相互作用。保守的谷氨酸和亮氨酸残基(JAK1的E957和L959,JAK2的E930和L932,和位于铰链区的J903的E903和L905)通过强形成的氢键稳定了托法替尼的结合。与传入的托法替尼的络合导致ATP结合位点的封闭构象,并降低了JAK蛋白甘氨酸环上的蛋白质波动。托法替尼/ JAKs的结合亲和力的排列顺序为JAK3> JAK2〜JAK1,与报道的实验数据一致。
更新日期:2020-01-14
中文翻译:

深入了解Tofacitinib对Janus激酶的结合识别和敏感性。
Janus激酶(JAKs)是参与影响造血和免疫细胞功能的信号通路的酶。JAK1,JAK2和JAK3在多种免疫系统疾病中扮演不同的角色,也被认为是癌症治疗的潜在靶标。在本研究中,使用500 ns分子动力学(MD)模拟和基于MM-PB(GB)SA,QM / MM-GBSA的自由能计算,阐明了口服JAK抑制剂tofacitinib对这三种JAK的敏感性( PM3和SCC-DFTB)以及SIE方法。获得的结果表明托法替尼可以通过静电吸引,氢键形成,特别是范德华相互作用与ATP结合位点上的所有JAK相互作用。保守的谷氨酸和亮氨酸残基(JAK1的E957和L959,JAK2的E930和L932,和位于铰链区的J903的E903和L905)通过强形成的氢键稳定了托法替尼的结合。与传入的托法替尼的络合导致ATP结合位点的封闭构象,并降低了JAK蛋白甘氨酸环上的蛋白质波动。托法替尼/ JAKs的结合亲和力的排列顺序为JAK3> JAK2〜JAK1,与报道的实验数据一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号