Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Derivatives of DANPY (Dialkylaminonaphthylpyridinium), a DNA-Binding Fluorophore: Practical Synthesis of Tricyclic 2-Amino-6-bromonaphthalenes by Bucherer Reaction.
ACS Omega ( IF 3.7 ) Pub Date : 2019-12-23 , DOI: 10.1021/acsomega.9b03107 Jason S Kingsbury 1 , Delwin L Elder 2 , Lewis E Johnson 2 , Brittany A Smolarski 1 , Hannah E Zeitler 2 , Emily G Armbruster 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-12-23 , DOI: 10.1021/acsomega.9b03107 Jason S Kingsbury 1 , Delwin L Elder 2 , Lewis E Johnson 2 , Brittany A Smolarski 1 , Hannah E Zeitler 2 , Emily G Armbruster 1
Affiliation
|
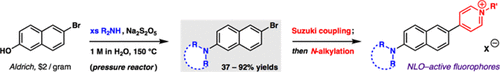
A simple and convergent way to synthesize 2-amino-6-bromonaphthalenes involves condensation of free secondary amines with the corresponding 2-naphthol under Bucherer conditions. The amination protocol relies on common Teflon-capped pressure flasks and has been used to modify the tertiary aminonaphthalene core of DANPY, a biocompatible chromophore shown to be safe and effective for staining a variety of cellular targets. Following a Suzuki reaction with pyridine 4-boronic acid, additional diversity is introduced upon N-alkylation to install the pyridinium cation. New DANPY derivatives and intermediates reported herein reflect the modularity of the dye nucleus, including the addition of groups useful for applications in membrane staining and DNA-based biophotonics.
中文翻译:

DANPY(二烷基氨基萘基吡啶鎓)的衍生物,一种 DNA 结合荧光团:通过 Bucherer 反应实际合成三环 2-氨基-6-溴萘。
合成 2-氨基-6-溴萘的一种简单而收敛的方法包括在 Bucherer 条件下将游离仲胺与相应的 2-萘酚缩合。胺化协议依赖于常见的聚四氟乙烯压力瓶,并已用于修改 DANPY 的叔氨基萘核心,DANPY 是一种生物相容性发色团,可安全有效地染色各种细胞靶标。在 Suzuki 与吡啶 4-硼酸反应之后,在 N-烷基化时引入了额外的多样性以安装吡啶鎓阳离子。本文报道的新 DANPY 衍生物和中间体反映了染料核的模块化,包括添加了可用于膜染色和基于 DNA 的生物光子学应用的基团。
更新日期:2020-01-14
中文翻译:

DANPY(二烷基氨基萘基吡啶鎓)的衍生物,一种 DNA 结合荧光团:通过 Bucherer 反应实际合成三环 2-氨基-6-溴萘。
合成 2-氨基-6-溴萘的一种简单而收敛的方法包括在 Bucherer 条件下将游离仲胺与相应的 2-萘酚缩合。胺化协议依赖于常见的聚四氟乙烯压力瓶,并已用于修改 DANPY 的叔氨基萘核心,DANPY 是一种生物相容性发色团,可安全有效地染色各种细胞靶标。在 Suzuki 与吡啶 4-硼酸反应之后,在 N-烷基化时引入了额外的多样性以安装吡啶鎓阳离子。本文报道的新 DANPY 衍生物和中间体反映了染料核的模块化,包括添加了可用于膜染色和基于 DNA 的生物光子学应用的基团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号