当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cell-Type-Specific CRISPR/Cas9 Delivery by Biomimetic Metal Organic Frameworks
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b11638 Mram Z Alyami 1 , Shahad K Alsaiari 1 , Yanyan Li 2 , Somayah S Qutub 1 , Fajr A Aleisa 2 , Rachid Sougrat 3 , Jasmeen S Merzaban 2 , Niveen M Khashab 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b11638 Mram Z Alyami 1 , Shahad K Alsaiari 1 , Yanyan Li 2 , Somayah S Qutub 1 , Fajr A Aleisa 2 , Rachid Sougrat 3 , Jasmeen S Merzaban 2 , Niveen M Khashab 1
Affiliation
|
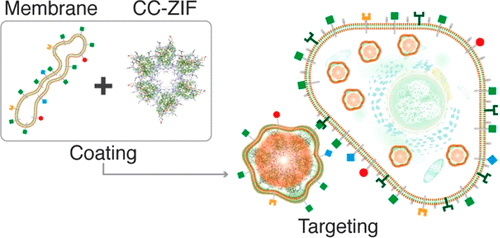
Effective and cell-type specific delivery of CRISPR/Cas9 gene editing elements remains a chal-lenging open problem. Here we report the devel-opment of biomimetic cancer cell coated zeolitic imidazolate frameworks (ZIFs) for targeted and cell-specific delivery of this genome editing ma-chinery. Coating ZIF-8 that is encapsulating CRISPR/Cas9 (CC-ZIF) with cancer cell membrane resulted in the uniformly covered C3-ZIF(cell membrane type). Incubation of C3-ZIFMCF with MCF-7, HeLa, HDFn and aTC cell lines showed the highest uptake by MCF-7 cells and negligible uptake by the healthy cells (i.e. HDFn and aTC). As to genome editing, a 3-fold repression in the EGFP expression was ob-served when MCF-7 were transfected with C3-ZIFMCF compared to 1-fold repression in the EGFP expres-sion when MCF-7 were transfected with C3-ZIFHELA. In-vivo testing confirmed the selectivity of C3-ZIFMCF to accumulate in MCF-7 tumor cells. This supports the ability of this biomimetic approach to match the needs of cell-specific targeting which is unquestionably the most critical step in the future translation of genome editing technologies.
中文翻译:

通过仿生金属有机框架进行细胞类型特异性 CRISPR/Cas9 递送
CRISPR/Cas9 基因编辑元件的有效和细胞类型特异性递送仍然是一个具有挑战性的开放性问题。在这里,我们报告了仿生癌细胞包被的沸石咪唑酯框架 (ZIF) 的开发,用于该基因组编辑机器的靶向和细胞特异性递送。用癌细胞膜包裹 CRISPR/Cas9 (CC-ZIF) 的 ZIF-8 导致均匀覆盖的 C3-ZIF(细胞膜类型)。C3-ZIFMCF 与 MCF-7、HeLa、HDFn 和 aTC 细胞系的孵育显示 MCF-7 细胞吸收最高,而健康细胞(即 HDFn 和 aTC)的吸收可忽略不计。关于基因组编辑,当 MCF-7 转染 C3-ZIFMCF 时,观察到 EGFP 表达的 3 倍抑制,而 MCF-7 转染 C3-ZIFHELA 时 EGFP 表达的 1 倍抑制. 体内测试证实了 C3-ZIFMCF 在 MCF-7 肿瘤细胞中积累的选择性。这支持了这种仿生方法能够满足细胞特异性靶向的需求,这无疑是未来基因组编辑技术转化中最关键的一步。
更新日期:2020-01-14
中文翻译:

通过仿生金属有机框架进行细胞类型特异性 CRISPR/Cas9 递送
CRISPR/Cas9 基因编辑元件的有效和细胞类型特异性递送仍然是一个具有挑战性的开放性问题。在这里,我们报告了仿生癌细胞包被的沸石咪唑酯框架 (ZIF) 的开发,用于该基因组编辑机器的靶向和细胞特异性递送。用癌细胞膜包裹 CRISPR/Cas9 (CC-ZIF) 的 ZIF-8 导致均匀覆盖的 C3-ZIF(细胞膜类型)。C3-ZIFMCF 与 MCF-7、HeLa、HDFn 和 aTC 细胞系的孵育显示 MCF-7 细胞吸收最高,而健康细胞(即 HDFn 和 aTC)的吸收可忽略不计。关于基因组编辑,当 MCF-7 转染 C3-ZIFMCF 时,观察到 EGFP 表达的 3 倍抑制,而 MCF-7 转染 C3-ZIFHELA 时 EGFP 表达的 1 倍抑制. 体内测试证实了 C3-ZIFMCF 在 MCF-7 肿瘤细胞中积累的选择性。这支持了这种仿生方法能够满足细胞特异性靶向的需求,这无疑是未来基因组编辑技术转化中最关键的一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号