当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On-surface dehydro-Diels-Alder reaction of dibromo-bis(phenylethynyl)benzene
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b11755 Marco Di Giovannantonio 1 , Ashok Keerthi 2, 3 , José I Urgel 1 , Martin Baumgarten 2 , Xinliang Feng 4 , Pascal Ruffieux 1 , Akimitsu Narita 2, 5 , Roman Fasel 1, 6 , Klaus Müllen 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-14 , DOI: 10.1021/jacs.9b11755 Marco Di Giovannantonio 1 , Ashok Keerthi 2, 3 , José I Urgel 1 , Martin Baumgarten 2 , Xinliang Feng 4 , Pascal Ruffieux 1 , Akimitsu Narita 2, 5 , Roman Fasel 1, 6 , Klaus Müllen 2
Affiliation
|
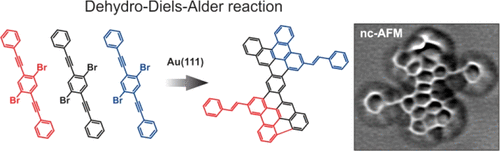
On-surface synthesis under ultrahigh vacuum conditions is a powerful tool to achieve molecular structures which cannot be accessed via traditional wet chemistry. Nevertheless, only a very limited number of chemical reac-tions out of the wide variety known from solution chemistry have been reported to proceed readily on atomically flat substrates. Cycloadditions are a class of reactions that are particularly important in the synthesis of sp2-hybridized carbon-based nanostructures. Here, we report on a specific type of [4+2] cycloaddition, namely a dehydro-Diels-Alder (DDA) reaction, performed between bis(phenylethynyl)-benzene precursors on Au(111). Unlike a Diels-Alder reaction, DDA exploits ethynyl groups to achieve the formation of an extra six-membered ring. Despite its extensive use in solution chemistry for more than a century, this reaction has never been reported to occur on surfaces. The specific choice of our precursor molecule has led to the successful synthesis of benzo- and naphtho-fused tetracene and heptacene products bearing styryl groups, as confirmed by scanning tunneling microscopy and noncontact atomic force microscopy. The two products arise from DDA dimerization and trimerization of the precursor molecules, respectively, and their observation opens perspectives to use DDA reactions as a novel on-surface synthesis tool.
中文翻译:

二溴双(苯基乙炔基)苯的表面脱氢-Diels-Alder反应
超高真空条件下的表面合成是实现传统湿化学无法获得的分子结构的有力工具。然而,据报道,在溶液化学中已知的种类繁多的化学反应中,只有非常有限数量的化学反应可以在原子平坦的基材上轻松进行。环加成反应是一类在 sp2 杂化碳基纳米结构合成中特别重要的反应。在这里,我们报告了一种特定类型的 [4+2] 环加成反应,即脱氢-狄尔斯-阿尔德 (DDA) 反应,在 Au(111) 上的双(苯乙炔基)-苯前体之间进行。与 Diels-Alder 反应不同,DDA 利用乙炔基来形成额外的六元环。尽管它在溶液化学中已被广泛使用了一个多世纪,从未报道过这种反应发生在表面上。正如扫描隧道显微镜和非接触式原子力显微镜所证实的那样,我们前体分子的特定选择导致成功合成了带有苯乙烯基的苯并和萘并四苯和七苯产品。这两种产物分别来自前体分子的 DDA 二聚化和三聚化,它们的观察为使用 DDA 反应作为一种新型的表面合成工具开辟了前景。
更新日期:2020-01-14
中文翻译:

二溴双(苯基乙炔基)苯的表面脱氢-Diels-Alder反应
超高真空条件下的表面合成是实现传统湿化学无法获得的分子结构的有力工具。然而,据报道,在溶液化学中已知的种类繁多的化学反应中,只有非常有限数量的化学反应可以在原子平坦的基材上轻松进行。环加成反应是一类在 sp2 杂化碳基纳米结构合成中特别重要的反应。在这里,我们报告了一种特定类型的 [4+2] 环加成反应,即脱氢-狄尔斯-阿尔德 (DDA) 反应,在 Au(111) 上的双(苯乙炔基)-苯前体之间进行。与 Diels-Alder 反应不同,DDA 利用乙炔基来形成额外的六元环。尽管它在溶液化学中已被广泛使用了一个多世纪,从未报道过这种反应发生在表面上。正如扫描隧道显微镜和非接触式原子力显微镜所证实的那样,我们前体分子的特定选择导致成功合成了带有苯乙烯基的苯并和萘并四苯和七苯产品。这两种产物分别来自前体分子的 DDA 二聚化和三聚化,它们的观察为使用 DDA 反应作为一种新型的表面合成工具开辟了前景。









































 京公网安备 11010802027423号
京公网安备 11010802027423号