Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Osocimab in Preventing Venous Thromboembolism Among Patients Undergoing Knee Arthroplasty
JAMA ( IF 63.1 ) Pub Date : 2020-01-14 , DOI: 10.1001/jama.2019.20687 Jeffrey I Weitz 1, 2 , Rupert Bauersachs 3, 4 , Bastian Becker 5 , Scott D Berkowitz 5 , Maria C S Freitas 5 , Michael R Lassen 6 , Carola Metzig 5 , Gary E Raskob 7
JAMA ( IF 63.1 ) Pub Date : 2020-01-14 , DOI: 10.1001/jama.2019.20687 Jeffrey I Weitz 1, 2 , Rupert Bauersachs 3, 4 , Bastian Becker 5 , Scott D Berkowitz 5 , Maria C S Freitas 5 , Michael R Lassen 6 , Carola Metzig 5 , Gary E Raskob 7
Affiliation
|
Importance
The efficacy of factor XIa inhibition for thromboprophylaxis is unknown. Osocimab is a long-acting, fully human monoclonal antibody that inhibits factor XIa. Objective
To compare different doses of osocimab with enoxaparin and apixaban for thromboprophylaxis in patients who have undergone knee arthroplasty. Design, Setting, and Participants
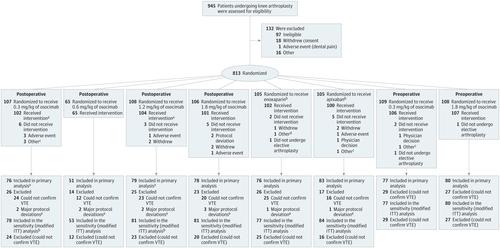
Randomized, open-label, adjudicator-blinded, phase 2 noninferiority trial with observer blinding for osocimab doses, conducted at 54 hospitals in 13 countries. Adult patients undergoing unilateral knee arthroplasty were randomized from October 2017 through August 2018 and followed up until January 2019. Interventions
Single intravenous osocimab postoperative doses of 0.3 mg/kg (n = 107), 0.6 mg/kg (n = 65), 1.2 mg/kg (n = 108), or 1.8 mg/kg (n = 106); preoperative doses of 0.3 mg/kg (n = 109) or 1.8 mg/kg (n = 108); or 40 mg of subcutaneous enoxaparin once daily (n = 105) or 2.5 mg of oral apixaban twice daily (n = 105) for at least 10 days or until venography. Main Outcomes and Measures
The primary outcome was venous thromboembolism incidence between 10 and 13 days postoperatively (assessed by mandatory bilateral venography performed 10 to 13 days after surgery or confirmed symptomatic deep vein thrombosis or pulmonary embolism). A 5% noninferiority margin compared with enoxaparin was chosen. The primary safety outcome of major or clinically relevant nonmajor bleeding was assessed until 10 to 13 days postoperatively. Results
Of 813 randomized participants (mean [SD] age, 66.5 years [8.2 years]; body mass index, 32.7 [5.7]; and 74.2% women), 600 were included in the per-protocol population used for the primary analysis. The primary outcome occurred in 18 patients (23.7%) receiving 0.3 mg/kg, 8 (15.7%) receiving 0.6 mg/kg, 13 (16.5%) receiving 1.2 mg/kg, and 14 (17.9%) receiving 1.8 mg/kg of osocimab postoperatively; 23 (29.9%) receiving 0.3 mg/kg and 9 (11.3%) receiving 1.8 mg/kg of osocimab preoperatively; 20 (26.3%) receiving enoxaparin; and 12 (14.5%) receiving apixaban. Osocimab given postoperatively met criteria for noninferiority compared with enoxaparin with risk differences (1-sided 95% CIs) of 10.6% (95% CI, -1.2% to ∞) at the 0.6-mg/kg dose; 9.9% (95% CI, -0.9% to ∞) at the 1.2-mg/kg dose, and 8.4% (95% CI, -2.6 to ∞) at the 1.8-mg/kg dose. The preoperative dose of 1.8 mg/kg of osocimab met criteria for superiority compared with enoxaparin with a risk difference of 15.1%; 2-sided 90% CI, 4.9% to 25.2%). Postoperative and preoperative doses of 0.3 mg/kg of osocimab did not meet the prespecified criteria for noninferiority, with risk differences (1-sided 95% CIs) of 2.6% (95% CI, -8.9% to ∞) and -3.6% (95% CI, -15.5% to ∞), respectively. Major or clinically relevant nonmajor bleeding was observed in up to 4.7% of those receiving osocimab, 5.9% receiving enoxaparin, and 2% receiving apixaban. Conclusions and Relevance
Among patients undergoing knee arthroplasty, postoperative osocimab 0.6 mg/kg, 1.2 mg/kg, and 1.8 mg/kg met criteria for noninferiority compared with enoxaparin, and the preoperative 1.8-mg/kg dose of osocimab met criteria for superiority compared with enoxaparin for the primary outcome of incidence of venous thromboembolism at 10 to 13 days postoperatively. Further studies are needed to establish efficacy and safety of osocimab relative to standard thromboprophylaxis. Trial Registration
ClinicalTrials.gov Identifier: NCT03276143.
中文翻译:

Osocimab 预防膝关节置换术患者静脉血栓栓塞的效果
重要性 XIa 因子抑制对血栓预防的功效尚不清楚。Osocimab 是一种长效、全人源单克隆抗体,可抑制因子 XIa。目的比较不同剂量的osocimab与依诺肝素和阿哌沙班在膝关节置换术患者血栓预防中的作用。设计、设置和参与者 在 13 个国家/地区的 54 家医院进行的随机、开放标签、裁判员盲法的 2 期非劣效性试验,观察者对 osocimab 剂量进行盲法。接受单侧膝关节置换术的成年患者从 2017 年 10 月至 2018 年 8 月随机分组,随访至 2019 年 1 月。 干预 单次静脉注射 osocimab 术后剂量为 0.3 mg/kg (n = 107)、0.6 mg/kg (n = 65)、1.2 mg /kg (n = 108),或 1.8 mg/kg (n = 106);术前剂量为 0.3 mg/kg (n = 109) 或 1。8 毫克/公斤 (n = 108); 或 40 mg 皮下依诺肝素每天一次 (n = 105) 或 2.5 mg 口服阿哌沙班每天两次 (n = 105) 至少 10 天或直至静脉造影。主要结果和措施 主要结果是术后 10 至 13 天的静脉血栓栓塞发生率(通过在手术后 10 至 13 天进行的强制性双侧静脉造影或确诊的有症状的深静脉血栓形成或肺栓塞进行评估)。与依诺肝素相比,选择了 5% 的非劣效性界限。在术后 10 至 13 天评估主要或临床相关的非大出血的主要安全性结果。结果 在 813 名随机参与者(平均 [SD] 年龄,66.5 岁 [8.2 岁];体重指数,32.7 [5.7];和 74.2% 女性)中,600 人被纳入用于主要分析的符合方案人群。主要结果发生在 18 名患者 (23.7%) 接受 0.3 mg/kg、8 (15.7%) 接受 0.6 mg/kg、13 (16.5%) 接受 1.2 mg/kg 和 14 (17.9%) 接受 1.8 mg/kg osocimab 术后;23 (29.9%) 名接受 0.3 mg/kg 和 9 (11.3%) 接受 1.8 mg/kg osocimab 术前;20 (26.3%) 名接受依诺肝素;和 12 (14.5%) 接受阿哌沙班。与依诺肝素相比,术后给予 Osocimab 符合非劣效性标准,0.6-mg/kg 剂量的风险差异(单侧 95% CI)为 10.6%(95% CI,-1.2% 至∞);1.2-mg/kg 剂量时为 9.9%(95% CI,-0.9% 至 ∞),1.8-mg/kg 剂量时为 8.4%(95% CI,-2.6 至 ∞)。与依诺肝素相比,术前 1.8 mg/kg 的 osocimab 剂量符合优越性标准,风险差异为 15.1%;2 侧 90% CI,4.9% 至 25.2%)。术后和术前剂量为 0。3 mg/kg osocimab 不符合预先指定的非劣效性标准,风险差异(单侧 95% CI)为 2.6%(95% CI,-8.9% 至∞)和 -3.6%(95% CI,- 15.5% 到∞),分别。在高达 4.7% 的接受 osocimab、5.9% 接受依诺肝素和 2% 接受阿哌沙班的患者中观察到大出血或临床相关的非大出血。结论和相关性 在接受膝关节置换术的患者中,与依诺肝素相比,术后 osocimab 0.6 mg/kg、1.2 mg/kg 和 1.8 mg/kg 符合非劣效性标准,术前 1.8 mg/kg 剂量 osocimab 满足优越性标准相比术后 10 至 13 天静脉血栓栓塞发生率的主要结局指标为依诺肝素。需要进一步的研究来确定 osocimab 相对于标准血栓预防的有效性和安全性。
更新日期:2020-01-14
中文翻译:

Osocimab 预防膝关节置换术患者静脉血栓栓塞的效果
重要性 XIa 因子抑制对血栓预防的功效尚不清楚。Osocimab 是一种长效、全人源单克隆抗体,可抑制因子 XIa。目的比较不同剂量的osocimab与依诺肝素和阿哌沙班在膝关节置换术患者血栓预防中的作用。设计、设置和参与者 在 13 个国家/地区的 54 家医院进行的随机、开放标签、裁判员盲法的 2 期非劣效性试验,观察者对 osocimab 剂量进行盲法。接受单侧膝关节置换术的成年患者从 2017 年 10 月至 2018 年 8 月随机分组,随访至 2019 年 1 月。 干预 单次静脉注射 osocimab 术后剂量为 0.3 mg/kg (n = 107)、0.6 mg/kg (n = 65)、1.2 mg /kg (n = 108),或 1.8 mg/kg (n = 106);术前剂量为 0.3 mg/kg (n = 109) 或 1。8 毫克/公斤 (n = 108); 或 40 mg 皮下依诺肝素每天一次 (n = 105) 或 2.5 mg 口服阿哌沙班每天两次 (n = 105) 至少 10 天或直至静脉造影。主要结果和措施 主要结果是术后 10 至 13 天的静脉血栓栓塞发生率(通过在手术后 10 至 13 天进行的强制性双侧静脉造影或确诊的有症状的深静脉血栓形成或肺栓塞进行评估)。与依诺肝素相比,选择了 5% 的非劣效性界限。在术后 10 至 13 天评估主要或临床相关的非大出血的主要安全性结果。结果 在 813 名随机参与者(平均 [SD] 年龄,66.5 岁 [8.2 岁];体重指数,32.7 [5.7];和 74.2% 女性)中,600 人被纳入用于主要分析的符合方案人群。主要结果发生在 18 名患者 (23.7%) 接受 0.3 mg/kg、8 (15.7%) 接受 0.6 mg/kg、13 (16.5%) 接受 1.2 mg/kg 和 14 (17.9%) 接受 1.8 mg/kg osocimab 术后;23 (29.9%) 名接受 0.3 mg/kg 和 9 (11.3%) 接受 1.8 mg/kg osocimab 术前;20 (26.3%) 名接受依诺肝素;和 12 (14.5%) 接受阿哌沙班。与依诺肝素相比,术后给予 Osocimab 符合非劣效性标准,0.6-mg/kg 剂量的风险差异(单侧 95% CI)为 10.6%(95% CI,-1.2% 至∞);1.2-mg/kg 剂量时为 9.9%(95% CI,-0.9% 至 ∞),1.8-mg/kg 剂量时为 8.4%(95% CI,-2.6 至 ∞)。与依诺肝素相比,术前 1.8 mg/kg 的 osocimab 剂量符合优越性标准,风险差异为 15.1%;2 侧 90% CI,4.9% 至 25.2%)。术后和术前剂量为 0。3 mg/kg osocimab 不符合预先指定的非劣效性标准,风险差异(单侧 95% CI)为 2.6%(95% CI,-8.9% 至∞)和 -3.6%(95% CI,- 15.5% 到∞),分别。在高达 4.7% 的接受 osocimab、5.9% 接受依诺肝素和 2% 接受阿哌沙班的患者中观察到大出血或临床相关的非大出血。结论和相关性 在接受膝关节置换术的患者中,与依诺肝素相比,术后 osocimab 0.6 mg/kg、1.2 mg/kg 和 1.8 mg/kg 符合非劣效性标准,术前 1.8 mg/kg 剂量 osocimab 满足优越性标准相比术后 10 至 13 天静脉血栓栓塞发生率的主要结局指标为依诺肝素。需要进一步的研究来确定 osocimab 相对于标准血栓预防的有效性和安全性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号