当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the Proteomics Dark Regions by VAILase Cleavage at Aliphatic Amino Acids.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.analchem.9b05048 Binwen Sun 1, 2 , Zheyi Liu 1 , Zheng Fang 1, 2 , Wei Dong 3 , Yang Yu 4, 5 , Mingliang Ye 1 , Lin Liu 3 , Hongda Wang 4, 5 , Fangjun Wang 1, 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.analchem.9b05048 Binwen Sun 1, 2 , Zheyi Liu 1 , Zheng Fang 1, 2 , Wei Dong 3 , Yang Yu 4, 5 , Mingliang Ye 1 , Lin Liu 3 , Hongda Wang 4, 5 , Fangjun Wang 1, 2
Affiliation
|
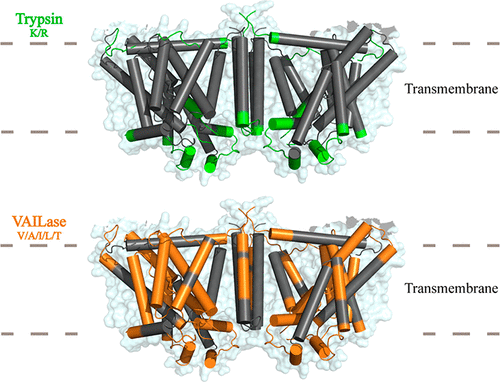
Proteomics emerges from the protein identification to protein functional elucidation, which depends to a large extent on the characterization of protein sequences. However, a large part of proteome sequences remains unannotated due to the limitation in proteolytic digestion by golden standard protease trypsin. Herein, we demonstrated that a cyanobacterial protease VAILase could specifically cleave at the short-chain aliphatic amino acids valine, alanine, leucine, isoleucine and threonine with cleavage specificity about 92% in total for proteomic analysis. The unique features of VAILase cleavage facilitate the characterization of most proteins and exhibit high complementarity to trypsin, and 22% of the covered sequences by VAILase are unique. VAILase can greatly improve the coverages of sequences with abundant aliphatic residues that are usually dark regions in conventional proteomic analysis, such as the transmembrane regions within anion exchanger 1 and photosystem II.
中文翻译:

通过在脂肪族氨基酸上的VAILase裂解来探测蛋白质组学的暗区。
蛋白质组学从蛋白质鉴定到蛋白质功能阐明,这在很大程度上取决于蛋白质序列的表征。然而,由于黄金标准蛋白酶胰蛋白酶对蛋白质水解的限制,蛋白质组序列的大部分仍未注释。在此,我们证明了蓝细菌蛋白酶VAILase可以特异性地切割短链脂肪族氨基酸缬氨酸,丙氨酸,亮氨酸,异亮氨酸和苏氨酸,其切割特异性总计约为92%,用于蛋白质组学分析。VAILase裂解的独特特征有助于大多数蛋白质的表征,并显示出与胰蛋白酶的高度互补性,并且VAILase覆盖的序列中22%是独特的。
更新日期:2020-01-14
中文翻译:

通过在脂肪族氨基酸上的VAILase裂解来探测蛋白质组学的暗区。
蛋白质组学从蛋白质鉴定到蛋白质功能阐明,这在很大程度上取决于蛋白质序列的表征。然而,由于黄金标准蛋白酶胰蛋白酶对蛋白质水解的限制,蛋白质组序列的大部分仍未注释。在此,我们证明了蓝细菌蛋白酶VAILase可以特异性地切割短链脂肪族氨基酸缬氨酸,丙氨酸,亮氨酸,异亮氨酸和苏氨酸,其切割特异性总计约为92%,用于蛋白质组学分析。VAILase裂解的独特特征有助于大多数蛋白质的表征,并显示出与胰蛋白酶的高度互补性,并且VAILase覆盖的序列中22%是独特的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号