当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Michael Addition Reaction Catalysed by Enantiopure Binuclear Nickel(II) Close‐Ended Helicates
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-28 , DOI: 10.1002/adsc.201901350 Eswaran Chinnaraja 1, 2 , Rajendran Arunachalam 1, 2 , Krishanu Samanta 2, 3 , Ramalingam Natarajan 2, 3 , Palani S. Subramanian 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-28 , DOI: 10.1002/adsc.201901350 Eswaran Chinnaraja 1, 2 , Rajendran Arunachalam 1, 2 , Krishanu Samanta 2, 3 , Ramalingam Natarajan 2, 3 , Palani S. Subramanian 1, 2
Affiliation
|
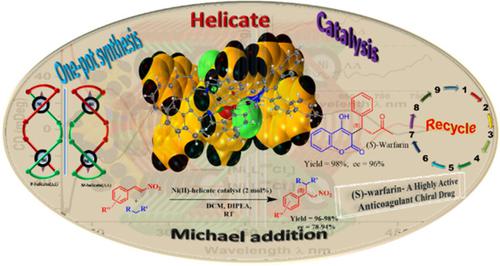
The enantiopure Ni(II) helicates [Ni2L1RR.Cl2] (1), [Ni2L1SS.Cl2] (1′), [Ni2L2RR.Cl2] (2), [Ni2L2SS.Cl2] (2′) were synthesized by one‐pot self‐assembly technique from R‐(+)‐ or S‐(−)‐1,1′‐binaphthyl‐2,2′‐diamine, with 4‐methyl‐2,6‐diformyl phenol or 4‐tert‐butyl‐2,6‐diformyl phenol and nickel salts. This binuclear double stranded Ni(II) helicates were characterized by ESI‐MS, IR and single crystal X‐ray structure wherever applicable. The extensive chiroptical studies suggest that the complexes are enantiopure in nature. The chirality transfer from ligand L1RR & L2RR to Ni(II) metal centre produced ΔΔ geometrical chirality, while their enantiomeric counterpart L1SS & L2SS produced ΛΛ chirality in their respective complexes.These enantiopure helicates were applied as catalysts in asymmetric Michael addition of 1,3‐dicarbonyl compounds with β‐nitrostyrene to produce nitroalkanes in good yield (96–98%) and ee (78–94%).
中文翻译:

对映纯双核镍(II)封闭端螺旋分子催化对映选择性迈克尔加成反应
的对映体纯的Ni(II)helicates [倪2大号1 RR .CL 2 ](1),[倪2大号1 SS .CL 2 ](1' ),[倪2大号2 RR .CL 2 ](2), [Ni 2 L 2 SS .Cl 2 ](2')是通过一锅自组装技术由R -(+)-或S -(-)-1,1'-联萘-2,2'-合成的二胺,与4-甲基-2,6-二甲酰基苯酚或4-叔丁基-2,6-丁基-二甲酰基苯酚和镍盐。这种双核双链Ni(II)螺旋结构在适用的情况下通过ESI-MS,IR和单晶X射线结构表征。大量的手性研究表明,该络合物在本质上是对映纯的。从配体L 1 RR和L 2 RR到Ni(II)金属中心的手性转移产生了ΔΔ几何手性,而它们的对映体对应物L 1 SS和L 2 SS 这些对映体纯的螺旋产物被用作催化剂,将1,3-二羰基化合物与β-硝基苯乙烯不对称迈克尔加成,以高产率(96-98%)和ee(78-94%)生产硝基烷。 。
更新日期:2020-01-29
中文翻译:

对映纯双核镍(II)封闭端螺旋分子催化对映选择性迈克尔加成反应
的对映体纯的Ni(II)helicates [倪2大号1 RR .CL 2 ](1),[倪2大号1 SS .CL 2 ](1' ),[倪2大号2 RR .CL 2 ](2), [Ni 2 L 2 SS .Cl 2 ](2')是通过一锅自组装技术由R -(+)-或S -(-)-1,1'-联萘-2,2'-合成的二胺,与4-甲基-2,6-二甲酰基苯酚或4-叔丁基-2,6-丁基-二甲酰基苯酚和镍盐。这种双核双链Ni(II)螺旋结构在适用的情况下通过ESI-MS,IR和单晶X射线结构表征。大量的手性研究表明,该络合物在本质上是对映纯的。从配体L 1 RR和L 2 RR到Ni(II)金属中心的手性转移产生了ΔΔ几何手性,而它们的对映体对应物L 1 SS和L 2 SS 这些对映体纯的螺旋产物被用作催化剂,将1,3-二羰基化合物与β-硝基苯乙烯不对称迈克尔加成,以高产率(96-98%)和ee(78-94%)生产硝基烷。 。









































 京公网安备 11010802027423号
京公网安备 11010802027423号