Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable immobilization of aldehyde ketone reductase mutants containing nonstandard amino acids on an epoxy resin via strain-promoted alkyne–azide cycloaddition
RSC Advances ( IF 3.9 ) Pub Date : 2020-1-14 , DOI: 10.1039/c9ra09067c Huimin Li 1 , Youcheng Yin 2 , Anming Wang 1 , Ningning Li 1 , Ru Wang 2 , Jing Zhang 1 , Xinxin Chen 1 , Xiaolin Pei 1 , Tian Xie 2
RSC Advances ( IF 3.9 ) Pub Date : 2020-1-14 , DOI: 10.1039/c9ra09067c Huimin Li 1 , Youcheng Yin 2 , Anming Wang 1 , Ningning Li 1 , Ru Wang 2 , Jing Zhang 1 , Xinxin Chen 1 , Xiaolin Pei 1 , Tian Xie 2
Affiliation
|
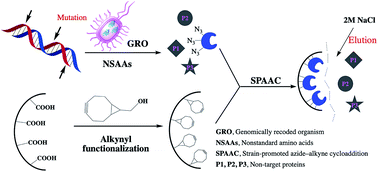
To avoid random chemical linkage and achieve precisely directed immobilization, mutant enzymes were obtained and immobilized using an incorporated reactive nonstandard amino acid (NSAA). For this purpose, aldehyde ketone reductase (AKR) was used as a model enzyme, and 110Y, 114Y, 143Y, 162Q and 189Q were each replaced with p-azido-L-phenylalanine (pAzF). Then, the mutant AKR was coupled to the functionalized support by strain-promoted alkyne–azide cycloaddition (SPAAC). The effects of the incorporation number and site of NSAAs on the loading and thermal stability of the immobilized AKR were examined. The results show that the mutant enzymes presented better specific activity than the wild type, except for AKR-110Y, and AKR-114Y showed 1.16-fold higher activity than the wild type. Moreover, the half-life (t1/2) of the five-point immobilized AKR reached 106 h and 45 h, 13 and 7 times higher than that of the free enzyme at 30 °C and 60 °C, respectively. Comparison of these three types of enzymes shows that multi-point immobilization provides improved loading and thermal stability and facilitates one-step purification. We expect this platform to facilitate a fundamental understanding of precisely oriented and controllable covalent immobilization and enable bio-manufacturing paradigms for fine chemicals and pharmaceuticals.
中文翻译:

通过应变促进的炔叠氮环加成将含有非标准氨基酸的醛酮还原酶突变体稳定固定在环氧树脂上
为了避免随机化学连接并实现精确定向固定,获得了突变酶并使用掺入的反应性非标准氨基酸(NSAA)进行固定。为此,使用醛酮还原酶(AKR)作为模型酶,并且将110Y、114Y、143Y、162Q和189Q各自替换为对叠氮基-L-苯丙氨酸(pAzF)。然后,通过菌株促进的炔叠氮环加成 (SPAAC) 将突变体 AKR 偶联到功能化载体上。研究了 NSAA 的掺入数量和位点对固定化 AKR 的负载量和热稳定性的影响。结果表明,除AKR-110Y外,突变酶比野生型表现出更好的比活性,AKR-114Y的活性比野生型高1.16倍。此外,五点固定化AKR的半衰期( t 1/2 )在30℃和60℃下分别达到106小时和45小时,分别比游离酶高13倍和7倍。这三种酶的比较表明,多点固定可提高负载和热稳定性,并有利于一步纯化。我们希望该平台能够促进对精确定向和可控共价固定化的基本理解,并实现精细化学品和药品的生物制造范例。
更新日期:2020-01-14
中文翻译:

通过应变促进的炔叠氮环加成将含有非标准氨基酸的醛酮还原酶突变体稳定固定在环氧树脂上
为了避免随机化学连接并实现精确定向固定,获得了突变酶并使用掺入的反应性非标准氨基酸(NSAA)进行固定。为此,使用醛酮还原酶(AKR)作为模型酶,并且将110Y、114Y、143Y、162Q和189Q各自替换为对叠氮基-L-苯丙氨酸(pAzF)。然后,通过菌株促进的炔叠氮环加成 (SPAAC) 将突变体 AKR 偶联到功能化载体上。研究了 NSAA 的掺入数量和位点对固定化 AKR 的负载量和热稳定性的影响。结果表明,除AKR-110Y外,突变酶比野生型表现出更好的比活性,AKR-114Y的活性比野生型高1.16倍。此外,五点固定化AKR的半衰期( t 1/2 )在30℃和60℃下分别达到106小时和45小时,分别比游离酶高13倍和7倍。这三种酶的比较表明,多点固定可提高负载和热稳定性,并有利于一步纯化。我们希望该平台能够促进对精确定向和可控共价固定化的基本理解,并实现精细化学品和药品的生物制造范例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号