当前位置:
X-MOL 学术
›
Cancer Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A multicenter phase II trial of paclitaxel, carboplatin, and cetuximab followed by chemoradiotherapy in patients with unresectable locally advanced squamous cell carcinoma of the head and neck.
Cancer Medicine ( IF 2.9 ) Pub Date : 2020-01-13 , DOI: 10.1002/cam4.2852 Tomohiro Enokida 1 , Takenori Ogawa 2 , Akihiro Homma 3 , Kenji Okami 4 , Shujiro Minami 5 , Ayako Nakanome 2 , Yasushi Shimizu 6 , Daisuke Maki 4 , Yuri Ueda 1 , Takao Fujisawa 1 , Atsushi Motegi 7 , Akira Ohkoshi 2 , Jun Taguchi 6 , Koji Ebisumoto 4 , Shogo Nomura 8 , Susumu Okano 1 , Makoto Tahara 1
Cancer Medicine ( IF 2.9 ) Pub Date : 2020-01-13 , DOI: 10.1002/cam4.2852 Tomohiro Enokida 1 , Takenori Ogawa 2 , Akihiro Homma 3 , Kenji Okami 4 , Shujiro Minami 5 , Ayako Nakanome 2 , Yasushi Shimizu 6 , Daisuke Maki 4 , Yuri Ueda 1 , Takao Fujisawa 1 , Atsushi Motegi 7 , Akira Ohkoshi 2 , Jun Taguchi 6 , Koji Ebisumoto 4 , Shogo Nomura 8 , Susumu Okano 1 , Makoto Tahara 1
Affiliation
|
BACKGROUND
Induction chemotherapy (IC) in locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN) often compromises compliance with subsequent chemoradiotherapy (CRT), which negatively affects outcomes. Here, we assessed the combination of paclitaxel (PTX), carboplatin (CBDCA), and cetuximab (Cmab) as IC for unresectable LA-SCCHN.
METHODS
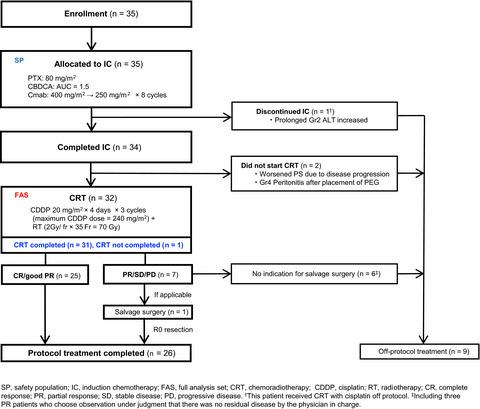
Induction chemotherapy consisted of weekly CBDCA area under the plasma concentration-time curve = 1.5, PTX 80 mg/m2 and Cmab with an initial dose of 400 mg/m2 followed by 250 mg/m2 for 8 weeks. Following IC, CDDP (20 mg/m2 , 4 days × 3 cycles) and concurrent radiotherapy (70 Gy/35 fr) were started. Primary endpoint was the proportion of CRT completion (%CRT completion). PCE was planned to be deemed effective if the Bayesian posterior probability (PP), defined as the probability that %CRT completion was larger than the threshold value of 65%, exceeded 84%.
RESULTS
Thirty-five patients were enrolled. Cases were hypopharynx/oropharynx/larynx in 17/17/1 patients, all at Stage IV. Of 35 patients, 34 (97%) completed IC and 32 received CRT and met the criteria of full analysis set (FAS). In FAS, the %CRT completion was 96.9%, and PP was 99.9%, exceeding the prespecified boundary of 84%. Mean cumulative dose and relative to dose intensity of CDDP in CRT was 232.5 mg/m2 and 100%, respectively. Response rate was 88.6% by IC and 93.8% in the CRT phase. Three year overall survival was 83.5%. Main grade 3 toxicities included neutropenia (11.4%) and skin rash (5.7%) during IC; and oral mucositis (31.3%) and neutropenia (12.5%) during CRT. No grade 4 toxicity or treatment-related death was seen.
CONCLUSIONS
PCE as IC was feasible, with promising efficacy and no effect on compliance with subsequent CRT in unresectable LA-SCCHN.
中文翻译:

一项针对紫杉醇,卡铂和西妥昔单抗的多中心II期临床试验,然后对不能手术切除的局部晚期头颈部鳞状细胞癌患者进行放化疗。
背景技术在局部晚期头颈部鳞状细胞癌(LA-SCCHN)中的诱导化学疗法(IC)通常会损害对后续化学放疗(CRT)的依从性,这会对结果产生负面影响。在这里,我们评估了紫杉醇(PTX),卡铂(CBDCA)和西妥昔单抗(Cmab)的组合作为不可切除LA-SCCHN的IC。方法诱导化疗由每周血浆浓度-时间曲线下CBDCA面积= 1.5,PTX 80 mg / m2和Cmab组成,初始剂量为400 mg / m2,随后为250 mg / m2,共8周。继IC后,开始进行CDDP(20 mg / m2,4天×3个周期)和同时放疗(70 Gy / 35 fr)。主要终点指标是CRT完成的比例(%CRT完成)。如果贝叶斯后验概率(PP)定义为%CRT完成率大于阈值65%超过84%的概率。结果招募了35例患者。17/17/1患者的下咽/口咽/喉部病例均为IV期。在35例患者中,有34例(97%)完成了IC,32例接受了CRT并符合完整分析集(FAS)的标准。在FAS中,CRT的完成率为96.9%,PP为99.9%,超出了规定的84%范围。CRT中CDDP的平均累积剂量和相对剂量强度分别为232.5 mg / m2和100%。IC的响应率为88.6%,CRT阶段的响应率为93.8%。三年总生存率为83.5%。IC期间主要的3级毒性包括中性粒细胞减少(11.4%)和皮疹(5.7%)。CRT期间口腔粘膜炎(31.3%)和中性粒细胞减少症(12.5%)。没有发现4级毒性或与治疗相关的死亡。
更新日期:2020-01-14
中文翻译:

一项针对紫杉醇,卡铂和西妥昔单抗的多中心II期临床试验,然后对不能手术切除的局部晚期头颈部鳞状细胞癌患者进行放化疗。
背景技术在局部晚期头颈部鳞状细胞癌(LA-SCCHN)中的诱导化学疗法(IC)通常会损害对后续化学放疗(CRT)的依从性,这会对结果产生负面影响。在这里,我们评估了紫杉醇(PTX),卡铂(CBDCA)和西妥昔单抗(Cmab)的组合作为不可切除LA-SCCHN的IC。方法诱导化疗由每周血浆浓度-时间曲线下CBDCA面积= 1.5,PTX 80 mg / m2和Cmab组成,初始剂量为400 mg / m2,随后为250 mg / m2,共8周。继IC后,开始进行CDDP(20 mg / m2,4天×3个周期)和同时放疗(70 Gy / 35 fr)。主要终点指标是CRT完成的比例(%CRT完成)。如果贝叶斯后验概率(PP)定义为%CRT完成率大于阈值65%超过84%的概率。结果招募了35例患者。17/17/1患者的下咽/口咽/喉部病例均为IV期。在35例患者中,有34例(97%)完成了IC,32例接受了CRT并符合完整分析集(FAS)的标准。在FAS中,CRT的完成率为96.9%,PP为99.9%,超出了规定的84%范围。CRT中CDDP的平均累积剂量和相对剂量强度分别为232.5 mg / m2和100%。IC的响应率为88.6%,CRT阶段的响应率为93.8%。三年总生存率为83.5%。IC期间主要的3级毒性包括中性粒细胞减少(11.4%)和皮疹(5.7%)。CRT期间口腔粘膜炎(31.3%)和中性粒细胞减少症(12.5%)。没有发现4级毒性或与治疗相关的死亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号