当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk After Acute Coronary Syndrome
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jacc.2019.10.057 Vera A Bittner 1 , Michael Szarek 2 , Philip E Aylward 3 , Deepak L Bhatt 4 , Rafael Diaz 5 , Jay M Edelberg 6 , Zlatko Fras 7 , Shaun G Goodman 8 , Sigrun Halvorsen 9 , Corinne Hanotin 10 , Robert A Harrington 11 , J Wouter Jukema 12 , Virginie Loizeau 10 , Patrick M Moriarty 13 , Angèle Moryusef 6 , Robert Pordy 14 , Matthew T Roe 15 , Peter Sinnaeve 16 , Sotirios Tsimikas 17 , Robert Vogel 18 , Harvey D White 19 , Doron Zahger 20 , Andreas M Zeiher 21 , Ph Gabriel Steg 22 , Gregory G Schwartz 18 ,
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jacc.2019.10.057 Vera A Bittner 1 , Michael Szarek 2 , Philip E Aylward 3 , Deepak L Bhatt 4 , Rafael Diaz 5 , Jay M Edelberg 6 , Zlatko Fras 7 , Shaun G Goodman 8 , Sigrun Halvorsen 9 , Corinne Hanotin 10 , Robert A Harrington 11 , J Wouter Jukema 12 , Virginie Loizeau 10 , Patrick M Moriarty 13 , Angèle Moryusef 6 , Robert Pordy 14 , Matthew T Roe 15 , Peter Sinnaeve 16 , Sotirios Tsimikas 17 , Robert Vogel 18 , Harvey D White 19 , Doron Zahger 20 , Andreas M Zeiher 21 , Ph Gabriel Steg 22 , Gregory G Schwartz 18 ,
Affiliation
|
BACKGROUND
Lipoprotein(a) concentration is associated with cardiovascular events. Alirocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, lowers lipoprotein(a) and low-density lipoprotein cholesterol (LDL-C). OBJECTIVES
A pre-specified analysis of the placebo-controlled ODYSSEY Outcomes trial in patients with recent acute coronary syndrome (ACS) determined whether alirocumab-induced changes in lipoprotein(a) and LDL-C independently predicted major adverse cardiovascular events (MACE). METHODS
One to 12 months after ACS, 18,924 patients on high-intensity statin therapy were randomized to alirocumab or placebo and followed for 2.8 years (median). Lipoprotein(a) was measured at randomization and 4 and 12 months thereafter. The primary MACE outcome was coronary heart disease death, nonfatal myocardial infarction, ischemic stroke, or hospitalization for unstable angina. RESULTS
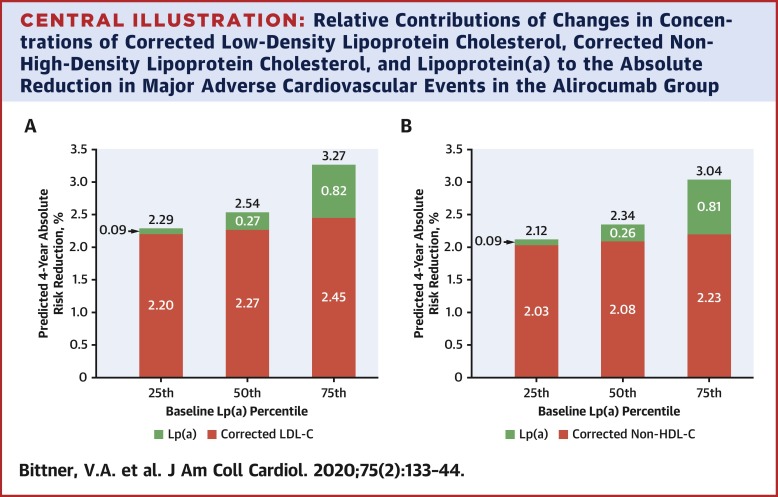
Baseline lipoprotein(a) levels (median: 21.2 mg/dl; interquartile range [IQR]: 6.7 to 59.6 mg/dl) and LDL-C [corrected for cholesterol content in lipoprotein(a)] predicted MACE. Alirocumab reduced lipoprotein(a) by 5.0 mg/dl (IQR: 0 to 13.5 mg/dl), corrected LDL-C by 51.1 mg/dl (IQR: 33.7 to 67.2 mg/dl), and reduced the risk of MACE (hazard ratio [HR]: 0.85; 95% confidence interval [CI]: 0.78 to 0.93). Alirocumab-induced reductions of lipoprotein(a) and corrected LDL-C independently predicted lower risk of MACE, after adjustment for baseline concentrations of both lipoproteins and demographic and clinical characteristics. A 1-mg/dl reduction in lipoprotein(a) with alirocumab was associated with a HR of 0.994 (95% CI: 0.990 to 0.999; p = 0.0081). CONCLUSIONS
Baseline lipoprotein(a) and corrected LDL-C levels and their reductions by alirocumab predicted the risk of MACE after recent ACS. Lipoprotein(a) lowering by alirocumab is an independent contributor to MACE reduction, which suggests that lipoprotein(a) should be an independent treatment target after ACS. (ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab; NCT01663402).
中文翻译:

Alirocumab 对急性冠脉综合征后脂蛋白 (a) 和心血管风险的影响
背景脂蛋白(a) 浓度与心血管事件有关。Alirocumab 是一种前蛋白转化酶枯草杆菌蛋白酶/kexin 9 型抑制剂,可降低脂蛋白 (a) 和低密度脂蛋白胆固醇 (LDL-C)。目标 一项针对近期急性冠脉综合征 (ACS) 患者的安慰剂对照 ODYSSEY Outcomes 试验的预先指定分析确定了 alirocumab 诱导的脂蛋白 (a) 和 LDL-C 变化是否独立预测主要不良心血管事件 (MACE)。方法 ACS 后 1 至 12 个月,18,924 名接受高强度他汀类药物治疗的患者被随机分配至 alirocumab 或安慰剂组,并随访 2.8 年(中位数)。脂蛋白 (a) 在随机分组时以及之后的 4 个月和 12 个月进行测量。主要 MACE 结局是冠心病死亡、非致死性心肌梗死、缺血性中风、或因不稳定心绞痛住院。结果 基线脂蛋白 (a) 水平(中位数:21.2 mg/dl;四分位距 [IQR]:6.7 至 59.6 mg/dl)和 LDL-C [校正脂蛋白 (a) 中的胆固醇含量] 预测了 MACE。Alirocumab 将脂蛋白 (a) 降低了 5.0 mg/dl(IQR:0 至 13.5 mg/dl),将 LDL-C 校正了 51.1 mg/dl(IQR:33.7 至 67.2 mg/dl),并降低了 MACE 的风险(危害比率 [HR]:0.85;95% 置信区间 [CI]:0.78 至 0.93)。在对脂蛋白的基线浓度以及人口统计学和临床特征进行调整后,Alirocumab 诱导的脂蛋白 (a) 降低和校正的 LDL-C 独立预测了较低的 MACE 风险。alirocumab 脂蛋白 (a) 降低 1 mg/dl 与 HR 为 0.994(95% CI:0.990 至 0.999;p = 0.0081)相关。结论 基线脂蛋白 (a) 和校正的 LDL-C 水平及其通过 alirocumab 的降低预测了近期 ACS 后发生 MACE 的风险。alirocumab 降低脂蛋白 (a) 是降低 MACE 的独立因素,这表明脂蛋白 (a) 应该是 ACS 后的独立治疗目标。(ODYSSEY 结果:Alirocumab 治疗期间急性冠状动脉综合征后心血管结果的评估;NCT01663402)。
更新日期:2020-01-01
中文翻译:

Alirocumab 对急性冠脉综合征后脂蛋白 (a) 和心血管风险的影响
背景脂蛋白(a) 浓度与心血管事件有关。Alirocumab 是一种前蛋白转化酶枯草杆菌蛋白酶/kexin 9 型抑制剂,可降低脂蛋白 (a) 和低密度脂蛋白胆固醇 (LDL-C)。目标 一项针对近期急性冠脉综合征 (ACS) 患者的安慰剂对照 ODYSSEY Outcomes 试验的预先指定分析确定了 alirocumab 诱导的脂蛋白 (a) 和 LDL-C 变化是否独立预测主要不良心血管事件 (MACE)。方法 ACS 后 1 至 12 个月,18,924 名接受高强度他汀类药物治疗的患者被随机分配至 alirocumab 或安慰剂组,并随访 2.8 年(中位数)。脂蛋白 (a) 在随机分组时以及之后的 4 个月和 12 个月进行测量。主要 MACE 结局是冠心病死亡、非致死性心肌梗死、缺血性中风、或因不稳定心绞痛住院。结果 基线脂蛋白 (a) 水平(中位数:21.2 mg/dl;四分位距 [IQR]:6.7 至 59.6 mg/dl)和 LDL-C [校正脂蛋白 (a) 中的胆固醇含量] 预测了 MACE。Alirocumab 将脂蛋白 (a) 降低了 5.0 mg/dl(IQR:0 至 13.5 mg/dl),将 LDL-C 校正了 51.1 mg/dl(IQR:33.7 至 67.2 mg/dl),并降低了 MACE 的风险(危害比率 [HR]:0.85;95% 置信区间 [CI]:0.78 至 0.93)。在对脂蛋白的基线浓度以及人口统计学和临床特征进行调整后,Alirocumab 诱导的脂蛋白 (a) 降低和校正的 LDL-C 独立预测了较低的 MACE 风险。alirocumab 脂蛋白 (a) 降低 1 mg/dl 与 HR 为 0.994(95% CI:0.990 至 0.999;p = 0.0081)相关。结论 基线脂蛋白 (a) 和校正的 LDL-C 水平及其通过 alirocumab 的降低预测了近期 ACS 后发生 MACE 的风险。alirocumab 降低脂蛋白 (a) 是降低 MACE 的独立因素,这表明脂蛋白 (a) 应该是 ACS 后的独立治疗目标。(ODYSSEY 结果:Alirocumab 治疗期间急性冠状动脉综合征后心血管结果的评估;NCT01663402)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号