当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pre-Hospital Administration of Epinephrine in Pediatric Patients With Out-of-Hospital Cardiac Arrest
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jacc.2019.10.052 Tasuku Matsuyama 1 , Sho Komukai 2 , Junichi Izawa 3 , Koichiro Gibo 4 , Masashi Okubo 5 , Kosuke Kiyohara 6 , Takeyuki Kiguchi 7 , Taku Iwami 7 , Bon Ohta 1 , Tetsuhisa Kitamura 8
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jacc.2019.10.052 Tasuku Matsuyama 1 , Sho Komukai 2 , Junichi Izawa 3 , Koichiro Gibo 4 , Masashi Okubo 5 , Kosuke Kiyohara 6 , Takeyuki Kiguchi 7 , Taku Iwami 7 , Bon Ohta 1 , Tetsuhisa Kitamura 8
Affiliation
|
BACKGROUND
There is little evidence about pre-hospital advanced life support including epinephrine administration for pediatric out-of-hospital cardiac arrests (OHCAs). OBJECTIVES
This study aimed to assess the effect of pre-hospital epinephrine administration by emergency-medical-service (EMS) personnel for pediatric OHCA. METHODS
This nationwide population-based observational study in Japan enrolled pediatric patients age 8 to 17 years with OHCA between January 2007 and December 2016. Patients were sequentially matched with or without epinephrine during cardiac arrest using a risk-set matching based on time-dependent propensity score (probability of receiving epinephrine) calculated at each minute after initiation of cardiopulmonary resuscitation by EMS personnel. The primary endpoint was 1-month survival. Secondary endpoints were 1-month survival with favorable neurological outcome, defined as the cerebral performance category scale of 1 or 2, and pre-hospital return of spontaneous circulation (ROSC). RESULTS
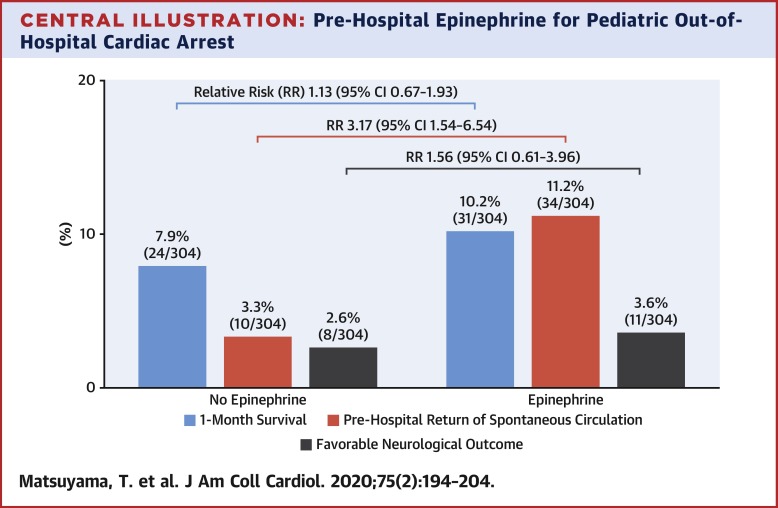
During the study period, a total of 1,214,658 OHCA patients were registered, and 3,961 pediatric OHCAs were eligible for analyses. Of these, 306 (7.7%) patients received epinephrine and 3,655 (92.3%) did not receive epinephrine. After time-dependent propensity score-sequential matching, 608 patients were included in the matched cohort. In the matched cohort, there were no significant differences between the epinephrine and no epinephrine groups in 1-month survival (epinephrine: 10.2% [31 of 304] vs. no epinephrine: 7.9% [24 of 304]; risk ratio [RR]: 1.13 [95% confidence interval (CI): 0.67 to 1.93]) and favorable neurological outcome (epinephrine: 3.6% [11 of 304] vs. no epinephrine: 2.6% [8 of 304]; RR: 1.56 [95% CI: 0.61 to 3.96]), whereas the epinephrine group had a higher likelihood of achieving pre-hospital ROSC (epinephrine: 11.2% [34 of 304] vs. no epinephrine: 3.3% [10 of 304]; RR: 3.17 [95% CI: 1.54 to 6.54]). CONCLUSIONS
In this study, pre-hospital epinephrine administration was associated with ROSC, whereas there were no significant differences in 1-month survival and favorable neurological outcome between those with and without epinephrine.
中文翻译:

院外心脏骤停患儿的院前肾上腺素给药
背景 几乎没有关于院前高级生命支持的证据,包括为儿科院外心脏骤停 (OHCA) 施用肾上腺素。目的 本研究旨在评估急诊医疗服务 (EMS) 人员院前注射肾上腺素对儿科 OHCA 的影响。方法 这项在日本进行的全国性基于人群的观察性研究在 2007 年 1 月至 2016 年 12 月期间招募了 8 至 17 岁患有 OHCA 的儿科患者。使用基于时间依赖性倾向的风险集匹配对患者在心脏骤停期间使用或不使用肾上腺素进行顺序匹配EMS人员开始心肺复苏后每分钟计算的评分(接受肾上腺素的概率)。主要终点是 1 个月的生存期。次要终点是具有良好神经学结果的 1 个月生存率,定义为脑功能分类量表 1 或 2,以及院前自主循环恢复(ROSC)。结果 在研究期间,共有 1,214,658 名 OHCA 患者注册,3,961 名儿科 OHCA 符合分析条件。其中,306 名 (7.7%) 患者接受肾上腺素治疗,3,655 名 (92.3%) 患者未接受肾上腺素治疗。经过时间依赖性倾向评分序列匹配后,匹配队列中包括 608 名患者。在匹配的队列中,肾上腺素组和非肾上腺素组之间的 1 个月生存率没有显着差异(肾上腺素:10.2% [304 中的 31] 与无肾上腺素:7.9% [304 中的 24];风险比 [RR] :1.13 [95% 置信区间 (CI):0.67 到 1。93]) 和良好的神经学结果(肾上腺素:3.6% [11 of 304] vs. 无肾上腺素:2.6% [8 of 304];RR:1.56 [95% CI:0.61 to 3.96]),而肾上腺素组有实现院前 ROSC 的可能性更高(肾上腺素:11.2% [34 of 304] 与无肾上腺素:3.3% [10 of 304];RR:3.17 [95% CI:1.54 至 6.54])。结论 在本研究中,院前使用肾上腺素与 ROSC 相关,而在使用和不使用肾上腺素的患者之间,1 个月的生存率和良好的神经系统结果没有显着差异。
更新日期:2020-01-01
中文翻译:

院外心脏骤停患儿的院前肾上腺素给药
背景 几乎没有关于院前高级生命支持的证据,包括为儿科院外心脏骤停 (OHCA) 施用肾上腺素。目的 本研究旨在评估急诊医疗服务 (EMS) 人员院前注射肾上腺素对儿科 OHCA 的影响。方法 这项在日本进行的全国性基于人群的观察性研究在 2007 年 1 月至 2016 年 12 月期间招募了 8 至 17 岁患有 OHCA 的儿科患者。使用基于时间依赖性倾向的风险集匹配对患者在心脏骤停期间使用或不使用肾上腺素进行顺序匹配EMS人员开始心肺复苏后每分钟计算的评分(接受肾上腺素的概率)。主要终点是 1 个月的生存期。次要终点是具有良好神经学结果的 1 个月生存率,定义为脑功能分类量表 1 或 2,以及院前自主循环恢复(ROSC)。结果 在研究期间,共有 1,214,658 名 OHCA 患者注册,3,961 名儿科 OHCA 符合分析条件。其中,306 名 (7.7%) 患者接受肾上腺素治疗,3,655 名 (92.3%) 患者未接受肾上腺素治疗。经过时间依赖性倾向评分序列匹配后,匹配队列中包括 608 名患者。在匹配的队列中,肾上腺素组和非肾上腺素组之间的 1 个月生存率没有显着差异(肾上腺素:10.2% [304 中的 31] 与无肾上腺素:7.9% [304 中的 24];风险比 [RR] :1.13 [95% 置信区间 (CI):0.67 到 1。93]) 和良好的神经学结果(肾上腺素:3.6% [11 of 304] vs. 无肾上腺素:2.6% [8 of 304];RR:1.56 [95% CI:0.61 to 3.96]),而肾上腺素组有实现院前 ROSC 的可能性更高(肾上腺素:11.2% [34 of 304] 与无肾上腺素:3.3% [10 of 304];RR:3.17 [95% CI:1.54 至 6.54])。结论 在本研究中,院前使用肾上腺素与 ROSC 相关,而在使用和不使用肾上腺素的患者之间,1 个月的生存率和良好的神经系统结果没有显着差异。










































 京公网安备 11010802027423号
京公网安备 11010802027423号