当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Reversal Cyclization To Access either Eight-Membered Sulfur-Containing Heterocycle-Fused γ-Pyrones or 2-(1,4-Dithianyl)-4-pyrones from the Same Precursors.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.joc.9b03303 Ling Tang 1, 2 , Qin Yang 1, 2 , Jianfang Zhang 1, 2 , Guisheng Deng 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.joc.9b03303 Ling Tang 1, 2 , Qin Yang 1, 2 , Jianfang Zhang 1, 2 , Guisheng Deng 1, 2
Affiliation
|
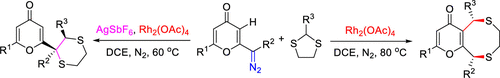
A regioselective reverse strategy for the construction of eight-membered sulfur-containing heterocycle-fused γ-pyrones and 2-(1,4-dithianyl)-4-pyrones starting from 2-diazo-γ-pyrones and dithioacetals was achieved for the first time. The process combines C-S bond formation via sulfur ylides and C-C bond formation via electron transfer to afford the target molecules in a facile manner with 100% regioselectivity and in excellent isolated yields (up to 90%).
中文翻译:

区域选择性反向环化可从相同的前体中获得八元含硫杂环杂环γ-吡喃酮或2-(1,4-二噻吩基)-4-吡喃酮。
第一个实现了从2-重氮-γ-吡喃酮和二硫缩醛开始构建八元含硫杂环稠合的γ-吡喃酮和2-(1,4-二硫烷基)-4-吡喃酮的区域选择性逆向策略。时间。该方法结合了通过硫酰化物形成的CS键和通过电子转移形成的CC键,以简便的方式提供目标分子,具有100%的区域选择性和极佳的分离产率(最高90%)。
更新日期:2020-01-23
中文翻译:

区域选择性反向环化可从相同的前体中获得八元含硫杂环杂环γ-吡喃酮或2-(1,4-二噻吩基)-4-吡喃酮。
第一个实现了从2-重氮-γ-吡喃酮和二硫缩醛开始构建八元含硫杂环稠合的γ-吡喃酮和2-(1,4-二硫烷基)-4-吡喃酮的区域选择性逆向策略。时间。该方法结合了通过硫酰化物形成的CS键和通过电子转移形成的CC键,以简便的方式提供目标分子,具有100%的区域选择性和极佳的分离产率(最高90%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号