当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoelectronic effects impact glycan recognition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-13 , DOI: 10.1021/jacs.9b11699 Caitlin M McMahon 1 , Christine R Isabella 1 , Ian W Windsor 1 , Paul Kosma 2 , Ronald T Raines 1 , Laura L Kiessling 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-13 , DOI: 10.1021/jacs.9b11699 Caitlin M McMahon 1 , Christine R Isabella 1 , Ian W Windsor 1 , Paul Kosma 2 , Ronald T Raines 1 , Laura L Kiessling 1
Affiliation
|
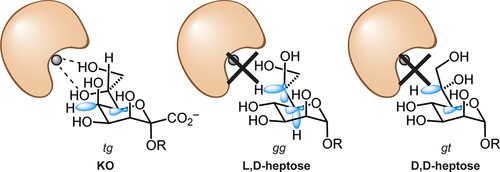
Recognition of distinct glycans is central to biology, and lectins mediate this function. Lectin glycan preferences are usually centered on specific monosaccharides. In contrast, human intelectin-1 (hItln-1, also known as Omentin-1) is a soluble lectin that binds a range of microbial sugars, including β-D-galactofuranose (β-Galf), D-glycerol 1-phosphate, D-glycero-D-talo-oct-2-ulosonic acid (KO), and 3-deoxy-D-manno-oct-2-ulosonic acid (KDO). Though these saccharides differ dramatically in structure, they share a common feature-an exocyclic vicinal diol. How and whether such a small fragment is sufficient for recognition was unclear. We tested several glycans with this epitope and found that L-glycero-α-D-manno-heptose and D-glycero-α-D-manno-heptose possess the critical diol motif yet bind weakly. To better understand hItln-1 recognition, we determined the structure of the hItln-1·KO complex using X-ray crystallography, and our 1.59-Å resolution structure enabled unambiguous assignment of the bound KO conformation. This carbohydrate conformation was present in >97% of the KDO/KO structures in the Protein Data Bank. Bioinformatic analysis revealed that KO and KDO adopt a common conformation, while heptoses prefer different conformers. The preferred conformers of KO and KDO favor hItln-1 engagement, but those of the heptoses do not. Natural bond orbital (NBO) calculations suggest these observed conformations, including the side chain orientations, are stabilized by not only steric but also stereoelectronic effects. Thus, our data highlight a role for stereoelectronic effects in dictating the specificity of glycan recognition by proteins. Finally, our finding that hItln-1 avoids binding prevalent glycans with a terminal 1,2 diol (e.g., NeuAc, and L-glycero-α-D-manno-heptose) suggests the lectin has evolved to recognize distinct bacterial species.
中文翻译:

立体电子效应影响聚糖识别
识别不同的聚糖是生物学的核心,而凝集素介导了这一功能。凝集素聚糖偏好通常集中在特定的单糖上。相比之下,人类智能蛋白-1(hItln-1,也称为 Omentin-1)是一种可溶性凝集素,可结合一系列微生物糖,包括 β-D-呋喃半乳糖(β-Galf)、D-甘油 1-磷酸、 D-甘油-D-talo-oct-2-ulosonic acid (KO) 和 3-deoxy-D-manno-oct-2-ulosonic acid (KDO)。虽然这些糖类在结构上有很大的不同,但它们有一个共同的特征——环外邻二醇。如此小的片段如何以及是否足以识别尚不清楚。我们用该表位测试了几种聚糖,发现 L-甘油-α-D-甘露糖-庚糖和 D-甘油-α-D-甘露糖-庚糖具有关键的二醇基序,但结合较弱。为了更好地理解 hItln-1 识别,我们使用 X 射线晶体学确定了 hItln-1·KO 复合物的结构,我们的 1.59 Å 分辨率结构能够明确指定结合的 KO 构象。这种碳水化合物构象存在于蛋白质数据库中 >97% 的 KDO/KO 结构中。生物信息学分析表明,KO 和 KDO 采用共同的构象,而庚糖则更喜欢不同的构象异构体。KO 和 KDO 的首选构象异构体有利于 hItln-1 的参与,但庚糖的构象却没有。自然键轨道 (NBO) 计算表明,这些观察到的构象,包括侧链方向,不仅通过空间效应而且通过立体电子效应稳定。因此,我们的数据强调了立体电子效应在决定蛋白质识别聚糖的特异性方面的作用。最后,
更新日期:2020-01-13
中文翻译:

立体电子效应影响聚糖识别
识别不同的聚糖是生物学的核心,而凝集素介导了这一功能。凝集素聚糖偏好通常集中在特定的单糖上。相比之下,人类智能蛋白-1(hItln-1,也称为 Omentin-1)是一种可溶性凝集素,可结合一系列微生物糖,包括 β-D-呋喃半乳糖(β-Galf)、D-甘油 1-磷酸、 D-甘油-D-talo-oct-2-ulosonic acid (KO) 和 3-deoxy-D-manno-oct-2-ulosonic acid (KDO)。虽然这些糖类在结构上有很大的不同,但它们有一个共同的特征——环外邻二醇。如此小的片段如何以及是否足以识别尚不清楚。我们用该表位测试了几种聚糖,发现 L-甘油-α-D-甘露糖-庚糖和 D-甘油-α-D-甘露糖-庚糖具有关键的二醇基序,但结合较弱。为了更好地理解 hItln-1 识别,我们使用 X 射线晶体学确定了 hItln-1·KO 复合物的结构,我们的 1.59 Å 分辨率结构能够明确指定结合的 KO 构象。这种碳水化合物构象存在于蛋白质数据库中 >97% 的 KDO/KO 结构中。生物信息学分析表明,KO 和 KDO 采用共同的构象,而庚糖则更喜欢不同的构象异构体。KO 和 KDO 的首选构象异构体有利于 hItln-1 的参与,但庚糖的构象却没有。自然键轨道 (NBO) 计算表明,这些观察到的构象,包括侧链方向,不仅通过空间效应而且通过立体电子效应稳定。因此,我们的数据强调了立体电子效应在决定蛋白质识别聚糖的特异性方面的作用。最后,











































 京公网安备 11010802027423号
京公网安备 11010802027423号