当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Cyclobutane-Fused Tetracyclic Scaffolds via Visible-Light Photocatalysis for Building Molecular Complexity
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-13 , DOI: 10.1021/jacs.9b12129 Martins S Oderinde 1 , Edna Mao 1 , Antonio Ramirez 2 , Joseph Pawluczyk 1 , Christine Jorge 3 , Lyndon A M Cornelius 1 , James Kempson 1 , Muthalagu Vetrichelvan 4 , Manivel Pitchai 4 , Anuradha Gupta 4 , Arun Kumar Gupta 4 , Nicholas A Meanwell 1 , Arvind Mathur 1 , T G Murali Dhar 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-13 , DOI: 10.1021/jacs.9b12129 Martins S Oderinde 1 , Edna Mao 1 , Antonio Ramirez 2 , Joseph Pawluczyk 1 , Christine Jorge 3 , Lyndon A M Cornelius 1 , James Kempson 1 , Muthalagu Vetrichelvan 4 , Manivel Pitchai 4 , Anuradha Gupta 4 , Arun Kumar Gupta 4 , Nicholas A Meanwell 1 , Arvind Mathur 1 , T G Murali Dhar 1
Affiliation
|
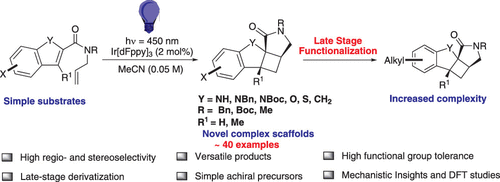
We describe the synthesis through visible-light photocatalysis of novel functionalized tetracyclic scaffolds that incorporate a fused azabicyclo[3.2.0]heptan-2-one motif, which are structurally interesting cores with potential in natural product synthesis and drug discovery. The synthetic approach involves an intramolecular [2+2] cycloaddition with concomitant dearomatization of the heterocycle via an energy transfer process promoted by an iridium-based photosensitizer, to build a complex molecular archi-tecture with three stereogenic centers from relatively simple, achiral precursors. These fused azabicyclo[3.2.0]heptan-2-one-based tetracycles were obtained in high yield (generally >99%) and with excellent diastereoselectivity (>99:1). The late-stage derivatization of a bromine-substituted, tetracyclic indoline derivative with alkyl groups, employing a mild Negishi C-C bond forming protocol as a means of increasing structural diversity, provides additional modularity that will enable the delivery of valuable building blocks for medicinal chemistry. DFT calculations were used to compute the T1-S0 free energy gap of the ole-fin-tethered precursors and also to predict their reactivities based on triplet state energy transfer and transition state energy feasi-bility.
中文翻译:

通过可见光光催化合成环丁烷稠合四环支架以构建分子复杂性
我们描述了通过可见光光催化合成新型功能化四环支架,这些支架结合了一个融合的氮杂双环 [3.2.0] 庚烷-2-one 基序,这些基序是结构有趣的核心,在天然产物合成和药物发现中具有潜力。合成方法涉及分子内 [2+2] 环加成反应,同时通过铱基光敏剂促进的能量转移过程使杂环脱芳构化,以从相对简单的非手性前体构建具有三个立体中心的复杂分子结构。这些基于稠合氮杂双环[3.2.0]庚烷-2-one的四环以高产率(通常> 99%)和优异的非对映选择性(> 99:1)获得。具有烷基的溴取代的四环二氢吲哚衍生物的后期衍生化,采用温和的 Negishi CC 键形成方案作为增加结构多样性的一种手段,提供了额外的模块化,能够为药物化学提供有价值的构建模块。DFT 计算用于计算烯烃系链前驱体的 T1-S0 自由能隙,并基于三重态能量转移和过渡态能量可行性预测其反应性。
更新日期:2020-01-13
中文翻译:

通过可见光光催化合成环丁烷稠合四环支架以构建分子复杂性
我们描述了通过可见光光催化合成新型功能化四环支架,这些支架结合了一个融合的氮杂双环 [3.2.0] 庚烷-2-one 基序,这些基序是结构有趣的核心,在天然产物合成和药物发现中具有潜力。合成方法涉及分子内 [2+2] 环加成反应,同时通过铱基光敏剂促进的能量转移过程使杂环脱芳构化,以从相对简单的非手性前体构建具有三个立体中心的复杂分子结构。这些基于稠合氮杂双环[3.2.0]庚烷-2-one的四环以高产率(通常> 99%)和优异的非对映选择性(> 99:1)获得。具有烷基的溴取代的四环二氢吲哚衍生物的后期衍生化,采用温和的 Negishi CC 键形成方案作为增加结构多样性的一种手段,提供了额外的模块化,能够为药物化学提供有价值的构建模块。DFT 计算用于计算烯烃系链前驱体的 T1-S0 自由能隙,并基于三重态能量转移和过渡态能量可行性预测其反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号