当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted degradation of anaplastic lymphoma kinase by gold nanoparticle-based multi-headed proteolysis targeting chimeras.
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.colsurfb.2020.110795 Yingming Wang 1 , Lingfei Han 1 , Fulei Liu 2 , Fubai Yang 3 , Xueyang Jiang 4 , Haopeng Sun 5 , Feng Feng 4 , Jingwei Xue 3 , Wenyuan Liu 1
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.colsurfb.2020.110795 Yingming Wang 1 , Lingfei Han 1 , Fulei Liu 2 , Fubai Yang 3 , Xueyang Jiang 4 , Haopeng Sun 5 , Feng Feng 4 , Jingwei Xue 3 , Wenyuan Liu 1
Affiliation
|
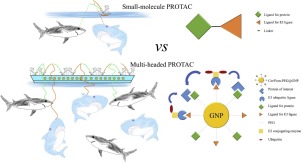
Anaplastic lymphoma kinase (ALK) is a major target in treating non-small-cell lung cancer, and several ALK inhibitors have been developed to antagonize its kinase activity. However, patients treated with inhibitors ultimately develop drug resistance. Therefore, therapies with new mechanisms of action are needed. Proteolysis targeting chimeras (PROTACs) are molecules that comprise a ligand for binding a protein of interest (POI), a connecting linker and a ligand for recruiting E3 ligase, and cause degradation of the target POI. Here, the first multi-headed PROTAC, as a proof of concept, is developed as a gold nanoparticle (GNP)-based drug delivery system for delivering PROTACs to target ALK. Pegylated GNPs loaded with both ceritinib and pomalidomide molecules, termed Cer/Pom-PEG@GNPs, showed good stability in several media. The GNP conjugates potently decreased the levels of ALK fusion proteins in a dose- and time-dependent manner, and specifically inhibited the proliferation of NCI-H2228 cells. In comparison with small molecule PROTACs, the new multi-headed PROTAC promoted the formation of coacervates of POIs/multi-headed PROTAC/E3 ubiquitin ligases, and POI and E3 ubiquitin ligase interacted through multidirectional ligands and a flexible linker, thereby avoiding the need for complicated structure optimization of PROTACs. In conclusion, Cer/Pom-PEG@GNPs can degrade intracellular ALK fusion proteins with minor off-target toxicity and can be applied in patients resistant to ALK inhibitors. As a nano-based drug carrier, Cer/Pom-PEG@GNPs have the potential to enable prolonged circulation and specifically distribute drugs to tumor regions in vivo; thus, further investigation is warranted.
中文翻译:

靶向嵌合体的基于金纳米粒子的多头蛋白水解靶向降解间变性淋巴瘤激酶。
间变性淋巴瘤激酶(ALK)是治疗非小细胞肺癌的主要靶标,并且已经开发了几种ALK抑制剂来拮抗其激酶活性。但是,用抑制剂治疗的患者最终会产生耐药性。因此,需要具有新作用机制的疗法。靶向嵌合体的蛋白水解(PROTAC)是包含用于结合目标蛋白(POI)的配体,连接接头和用于募集E3连接酶并引起目标POI降解的分子。在这里,作为概念证明,第一个多头PROTAC被开发为基于金纳米颗粒(GNP)的药物输送系统,用于将PROTAC输送至目标ALK。装载了赛立替尼和pomalidomide分子的聚乙二醇化GNP,称为Cer / Pom-PEG @ GNP,在几种培养基中均显示出良好的稳定性。GNP偶联物以剂量和时间依赖性方式有效降低了ALK融合蛋白的水平,并特异性抑制了NCI-H2228细胞的增殖。与小分子PROTAC相比,新的多头PROTAC促进了POI /多头PROTAC / E3泛素连接酶凝聚层的形成,并且POI和E3泛素连接酶通过多向配体和灵活的接头相互作用,从而避免了对PROTAC的复杂结构优化。总之,Cer / Pom-PEG @ GNPs可以降解细胞内ALK融合蛋白,并具有较小的脱靶毒性,可用于对ALK抑制剂耐药的患者。作为纳米基药物载体,Cer / Pom-PEG @ GNPs具有延长循环的潜力,并且可以将药物特异性地体内分布到肿瘤区域。从而,
更新日期:2020-01-14
中文翻译:

靶向嵌合体的基于金纳米粒子的多头蛋白水解靶向降解间变性淋巴瘤激酶。
间变性淋巴瘤激酶(ALK)是治疗非小细胞肺癌的主要靶标,并且已经开发了几种ALK抑制剂来拮抗其激酶活性。但是,用抑制剂治疗的患者最终会产生耐药性。因此,需要具有新作用机制的疗法。靶向嵌合体的蛋白水解(PROTAC)是包含用于结合目标蛋白(POI)的配体,连接接头和用于募集E3连接酶并引起目标POI降解的分子。在这里,作为概念证明,第一个多头PROTAC被开发为基于金纳米颗粒(GNP)的药物输送系统,用于将PROTAC输送至目标ALK。装载了赛立替尼和pomalidomide分子的聚乙二醇化GNP,称为Cer / Pom-PEG @ GNP,在几种培养基中均显示出良好的稳定性。GNP偶联物以剂量和时间依赖性方式有效降低了ALK融合蛋白的水平,并特异性抑制了NCI-H2228细胞的增殖。与小分子PROTAC相比,新的多头PROTAC促进了POI /多头PROTAC / E3泛素连接酶凝聚层的形成,并且POI和E3泛素连接酶通过多向配体和灵活的接头相互作用,从而避免了对PROTAC的复杂结构优化。总之,Cer / Pom-PEG @ GNPs可以降解细胞内ALK融合蛋白,并具有较小的脱靶毒性,可用于对ALK抑制剂耐药的患者。作为纳米基药物载体,Cer / Pom-PEG @ GNPs具有延长循环的潜力,并且可以将药物特异性地体内分布到肿瘤区域。从而,











































 京公网安备 11010802027423号
京公网安备 11010802027423号