当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of Active Site Architecture and Reaction Product Linkage Chemistry Reveals a Conserved Cleavage Substrate for an Endo-alpha-mannanase within Diverse Yeast Mannans.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.jmb.2019.12.048 Darryl R Jones 1 , Xiaohui Xing 1 , Jeffrey P Tingley 2 , Leeann Klassen 2 , Marissa L King 2 , Trevor W Alexander 2 , D Wade Abbott 1
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.jmb.2019.12.048 Darryl R Jones 1 , Xiaohui Xing 1 , Jeffrey P Tingley 2 , Leeann Klassen 2 , Marissa L King 2 , Trevor W Alexander 2 , D Wade Abbott 1
Affiliation
|
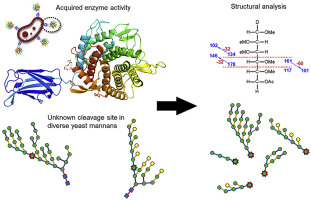
Yeast α-mannan (YM) is a densely branched N-linked glycan that decorates the surface of yeast cell walls. Owing to the high degree of branching, cleavage of the backbone of YM appears to rely on the coupled action of side-chain-cleaving enzymes. Upon examining the genome sequences of bovine-adapted Bacteroides thetaiotaomicron strains, isolated for their ability to degrade YM, we have identified a tandem pair of genes inserted into an orphan pathway predicted to be involved in YM metabolism. Here, we investigated the activity of one of these enzymes, a predicted endo-mannanase from glycoside hydrolase (GH) family 76 (BtGH76-MD40). Purified recombinant BtGH76-MD40 displayed activity on structurally distinct YMs from Saccharomyces cerevisiae and Schizosaccharomyces pombe. Linkage analysis of released oligosaccharide products from S. cerevisiae and S. pombe mannan determined BtGH76-MD40 targets a specific linkage that is conserved in structurally diverse YM substrates. In addition, using two differential derivatization methods, we have shown that there is an absolute requirement for undecorated d-mannopyranose in the -1 subsite. Determination of the BtGH76-MD40 X-ray crystal structure and structural superimposition and molecular docking of a branched alpha-mannopentatose substrate supported these findings. In contrast, BtGH76-MD40 can accommodate extended side chains in the +1 and -2 subsites, highlighting that a single alpha-1,6-mannosyl residue is a prerequisite for activity, and cleavage occurs at the reducing end of the undecorated monosaccharide. Collectively these results demonstrate how acquisition of new enzymes within extant pathways contributes to the functional abilities of saccharolytic bacteria persisting in complex digestive ecosystems.
中文翻译:

活性位点体系结构和反应产物连锁化学的分析揭示了酵母酵母甘露聚糖中内切α-甘露聚糖酶的保守裂解底物。
酵母α-甘露聚糖(YM)是一种密集分支的N-连接聚糖,可装饰酵母细胞壁的表面。由于高度的分支,YM主链的裂解似乎依赖于侧链裂解酶的偶联作用。检查牛适应的拟杆菌(Theactotaides thetaiotaomicron)菌株的基因组序列后,就其降解YM的能力进行了分离,我们确定了串联的一对基因,插入一对预测参与YM代谢的孤儿途径中。在这里,我们调查了其中一种酶的活性,一种糖苷水解酶(GH)家族76(BtGH76-MD40)预测的甘露聚糖内切甘露聚糖酶。纯化的重组BtGH76-MD40对酿酒酵母和粟酒裂殖酵母的结构不同YM表现出活性。酿酒酵母和酿酒酵母释放寡糖产物的连锁分析 Pombe甘露聚糖测定的BtGH76-MD40靶向在结构上不同的YM底物中保守的特定连接。此外,使用两种差分衍生方法,我们已经表明,在-1个子位点中对未修饰的d-甘露吡喃糖有绝对的要求。BtGH76-MD40 X射线晶体结构的确定以及支链α-甘露戊糖底物的结构重叠和分子对接支持了这些发现。相反,BtGH76-MD40可以在+1和-2亚位点容纳延伸的侧链,这突出表明单个α-1,6-甘露糖基残基是活性的先决条件,并且裂解发生在未修饰的单糖的还原端。
更新日期:2020-01-14
中文翻译:

活性位点体系结构和反应产物连锁化学的分析揭示了酵母酵母甘露聚糖中内切α-甘露聚糖酶的保守裂解底物。
酵母α-甘露聚糖(YM)是一种密集分支的N-连接聚糖,可装饰酵母细胞壁的表面。由于高度的分支,YM主链的裂解似乎依赖于侧链裂解酶的偶联作用。检查牛适应的拟杆菌(Theactotaides thetaiotaomicron)菌株的基因组序列后,就其降解YM的能力进行了分离,我们确定了串联的一对基因,插入一对预测参与YM代谢的孤儿途径中。在这里,我们调查了其中一种酶的活性,一种糖苷水解酶(GH)家族76(BtGH76-MD40)预测的甘露聚糖内切甘露聚糖酶。纯化的重组BtGH76-MD40对酿酒酵母和粟酒裂殖酵母的结构不同YM表现出活性。酿酒酵母和酿酒酵母释放寡糖产物的连锁分析 Pombe甘露聚糖测定的BtGH76-MD40靶向在结构上不同的YM底物中保守的特定连接。此外,使用两种差分衍生方法,我们已经表明,在-1个子位点中对未修饰的d-甘露吡喃糖有绝对的要求。BtGH76-MD40 X射线晶体结构的确定以及支链α-甘露戊糖底物的结构重叠和分子对接支持了这些发现。相反,BtGH76-MD40可以在+1和-2亚位点容纳延伸的侧链,这突出表明单个α-1,6-甘露糖基残基是活性的先决条件,并且裂解发生在未修饰的单糖的还原端。











































 京公网安备 11010802027423号
京公网安备 11010802027423号