当前位置:
X-MOL 学术
›
Cancer Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Autologous CAR T-cell therapies supply chain: challenges and opportunities?
Cancer Gene Therapy ( IF 4.8 ) Pub Date : 2020-01-14 , DOI: 10.1038/s41417-019-0157-z Maria M Papathanasiou 1 , Christos Stamatis 2, 3 , Matthew Lakelin 4 , Suzanne Farid 2, 3 , Nigel Titchener-Hooker 2, 3 , Nilay Shah 1
Cancer Gene Therapy ( IF 4.8 ) Pub Date : 2020-01-14 , DOI: 10.1038/s41417-019-0157-z Maria M Papathanasiou 1 , Christos Stamatis 2, 3 , Matthew Lakelin 4 , Suzanne Farid 2, 3 , Nigel Titchener-Hooker 2, 3 , Nilay Shah 1
Affiliation
|
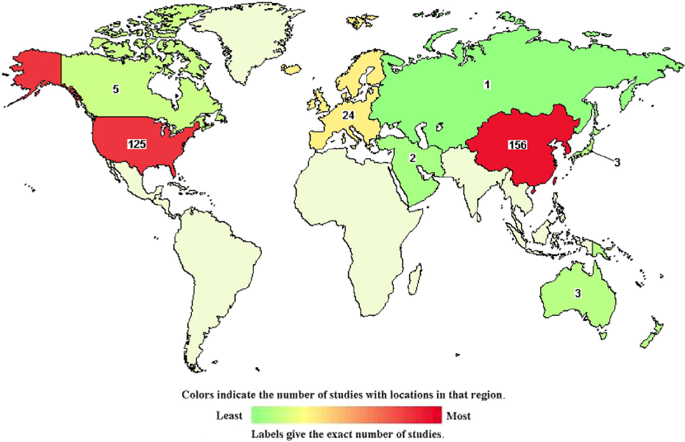
Chimeric antigen receptor (CAR) T cells are considered a potentially disruptive cancer therapy, showing highly promising results. Their recent success and regulatory approval (both in the USA and Europe) are likely to generate a rapidly increasing demand and a need for the design of robust and scalable manufacturing and distribution models that will ensure timely and cost-effective delivery of the therapy to the patient. However, there are challenging tasks as these therapies are accompanied by a series of constraints and particularities that need to be taken into consideration in the decision-making process. Here, we present an overview of the current state of the art in the CAR T cell market and present novel concepts that can debottleneck key elements of the current supply chain model and, we believe, help this technology achieve its long-term potential.
中文翻译:

自体 CAR T 细胞疗法供应链:挑战和机遇?
嵌合抗原受体 (CAR) T 细胞被认为是一种潜在的破坏性癌症疗法,显示出非常有希望的结果。它们最近的成功和监管部门的批准(在美国和欧洲)可能会产生快速增长的需求,并需要设计强大且可扩展的制造和分销模型,以确保及时且经济高效地向患者提供治疗病人。然而,由于这些疗法伴随着一系列需要在决策过程中考虑的限制和特殊性,因此存在具有挑战性的任务。在这里,我们概述了 CAR T 细胞市场的当前技术水平,并提出了可以消除当前供应链模型关键要素瓶颈的新概念,我们相信,
更新日期:2020-01-14
中文翻译:

自体 CAR T 细胞疗法供应链:挑战和机遇?
嵌合抗原受体 (CAR) T 细胞被认为是一种潜在的破坏性癌症疗法,显示出非常有希望的结果。它们最近的成功和监管部门的批准(在美国和欧洲)可能会产生快速增长的需求,并需要设计强大且可扩展的制造和分销模型,以确保及时且经济高效地向患者提供治疗病人。然而,由于这些疗法伴随着一系列需要在决策过程中考虑的限制和特殊性,因此存在具有挑战性的任务。在这里,我们概述了 CAR T 细胞市场的当前技术水平,并提出了可以消除当前供应链模型关键要素瓶颈的新概念,我们相信,











































 京公网安备 11010802027423号
京公网安备 11010802027423号