Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-01-13 , DOI: 10.1038/s41589-019-0444-x Matilde de Las Rivas 1 , Earnest James Paul Daniel 2 , Yoshiki Narimatsu 3 , Ismael Compañón 4 , Kentaro Kato 3, 5 , Pablo Hermosilla 6 , Aurélien Thureau 7 , Laura Ceballos-Laita 1 , Helena Coelho 8, 9 , Pau Bernadó 10 , Filipa Marcelo 8 , Lars Hansen 3 , Ryota Maeda 11 , Anabel Lostao 6, 12, 13 , Francisco Corzana 4 , Henrik Clausen 3 , Thomas A Gerken 2 , Ramon Hurtado-Guerrero 1, 3, 12
|
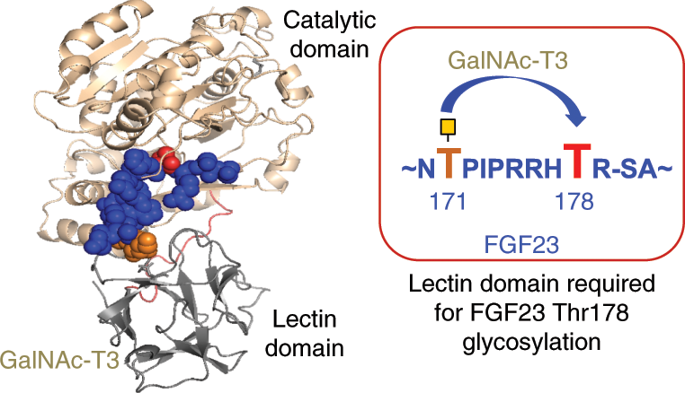
Polypeptide GalNAc-transferase T3 (GalNAc-T3) regulates fibroblast growth factor 23 (FGF23) by O-glycosylating Thr178 in a furin proprotein processing motif RHT178R↓S. FGF23 regulates phosphate homeostasis and deficiency in GALNT3 or FGF23 results in hyperphosphatemia and familial tumoral calcinosis. We explored the molecular mechanism for GalNAc-T3 glycosylation of FGF23 using engineered cell models and biophysical studies including kinetics, molecular dynamics and X-ray crystallography of GalNAc-T3 complexed to glycopeptide substrates. GalNAc-T3 uses a lectin domain mediated mechanism to glycosylate Thr178 requiring previous glycosylation at Thr171. Notably, Thr178 is a poor substrate site with limiting glycosylation due to substrate clashes leading to destabilization of the catalytic domain flexible loop. We suggest GalNAc-T3 specificity for FGF23 and its ability to control circulating levels of intact FGF23 is achieved by FGF23 being a poor substrate. GalNAc-T3’s structure further reveals the molecular bases for reported disease-causing mutations. Our findings provide an insight into how GalNAc-T isoenzymes achieve isoenzyme-specific nonredundant functions.
中文翻译:

GalNAc-T3 成纤维细胞生长因子 23 O-糖基化的分子基础
多肽 GalNAc 转移酶 T3 (GalNAc-T3) 通过对弗林蛋白酶原蛋白加工基序 RHT 178 R↓S 中的 Thr178 进行O -糖基化来调节成纤维细胞生长因子 23 (FGF23)。 FGF23 调节磷酸盐稳态, GALNT3或FGF23缺乏会导致高磷血症和家族性肿瘤钙质沉着症。我们利用工程细胞模型和生物物理学研究(包括与糖肽底物复合的 GalNAc-T3 的动力学、分子动力学和 X 射线晶体学)探索了 FGF23 GalNAc-T3 糖基化的分子机制。 GalNAc-T3 使用凝集素结构域介导的机制来糖基化 Thr178,需要在 Thr171 处进行先前的糖基化。值得注意的是,Thr178 是一个较差的底物位点,由于底物冲突导致催化结构域柔性环不稳定,糖基化受到限制。我们认为 GalNAc-T3 对 FGF23 的特异性及其控制完整 FGF23 循环水平的能力是通过 FGF23 作为不良底物来实现的。 GalNAc-T3 的结构进一步揭示了已报道的致病突变的分子基础。我们的研究结果提供了对 GalNAc-T 同工酶如何实现同工酶特异性非冗余功能的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号