当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Power-to-gas systems utilizing methanation reaction in solid oxide electrolysis cell cathodes: a model-based study
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9se00835g Naoya Fujiwara 1, 2, 3, 4 , Shohei Tada 1, 2, 3, 4 , Ryuji Kikuchi 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9se00835g Naoya Fujiwara 1, 2, 3, 4 , Shohei Tada 1, 2, 3, 4 , Ryuji Kikuchi 1, 2, 3, 4
Affiliation
|
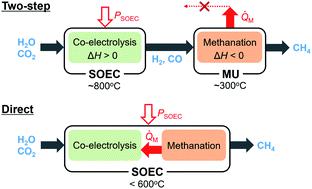
Power-to-gas is an emerging technology to convert electrical energy into chemical energy, and usually consists of two steps. First, hydrogen or syngas is produced in electrolysis cells, and then the hydrogen is used to methanate COx in catalytic reactors. Solid oxide electrolysis cells (SOECs) have the potential to integrate these two steps because methane can be directly synthesized in the SOEC cathodes. However, the advantages and drawbacks of such a direct process have not been discussed quantitatively. In the present work, we established a model using Aspen Plus to simulate the direct process. By analyzing the energy conversion processes in detail, it was clarified that the suppression of SOEC overpotentials at low temperatures is crucial. If the SOEC overpotentials are decreased at 400 °C, the energy conversion efficiency of the direct process will become higher than that of the conventional two-step process especially at small methane production rates. The superior performance can be ascribed to the recuperative heat utilization between the exothermic methanation reaction and the endothermic electrolysis reactions.
中文翻译:

在固体氧化物电解槽阴极中利用甲烷化反应的燃气系统:基于模型的研究
天然气发电是一种将电能转换为化学能的新兴技术,通常包括两个步骤。首先,在电解池中产生氢气或合成气,然后在催化反应器中使用氢气将CO x甲烷化。固态氧化物电解槽(SOEC)具有整合这两个步骤的潜力,因为甲烷可以直接在SOEC阴极中合成。但是,尚未直接讨论这种直接方法的优缺点。在当前的工作中,我们使用Aspen Plus建立了模型模拟直接过程。通过详细分析能量转换过程,可以弄清楚在低温下抑制SOEC超电势至关重要。如果SOEC的超电势在400°C时降低,则直接过程的能量转换效率将高于传统的两步过程,特别是在甲烷产量较低的情况下。优异的性能可以归因于放热的甲烷化反应和吸热的电解反应之间的换热利用。
更新日期:2020-01-13
中文翻译:

在固体氧化物电解槽阴极中利用甲烷化反应的燃气系统:基于模型的研究
天然气发电是一种将电能转换为化学能的新兴技术,通常包括两个步骤。首先,在电解池中产生氢气或合成气,然后在催化反应器中使用氢气将CO x甲烷化。固态氧化物电解槽(SOEC)具有整合这两个步骤的潜力,因为甲烷可以直接在SOEC阴极中合成。但是,尚未直接讨论这种直接方法的优缺点。在当前的工作中,我们使用Aspen Plus建立了模型模拟直接过程。通过详细分析能量转换过程,可以弄清楚在低温下抑制SOEC超电势至关重要。如果SOEC的超电势在400°C时降低,则直接过程的能量转换效率将高于传统的两步过程,特别是在甲烷产量较低的情况下。优异的性能可以归因于放热的甲烷化反应和吸热的电解反应之间的换热利用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号