当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical looping hydrogen storage and production: use of binary ferrite-spinel as oxygen carrier materials
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020/01/13 , DOI: 10.1039/c9se01104h Min Li 1, 2, 3, 4, 5 , Yu Qiu 1, 2, 3, 4, 5 , Li Ma 1, 2, 3, 4, 5 , Dongxu Cui 1, 2, 3, 4, 5 , Shuai Zhang 1, 2, 3, 4, 5 , Dewang Zeng 1, 2, 3, 4, 5 , Rui Xiao 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020/01/13 , DOI: 10.1039/c9se01104h Min Li 1, 2, 3, 4, 5 , Yu Qiu 1, 2, 3, 4, 5 , Li Ma 1, 2, 3, 4, 5 , Dongxu Cui 1, 2, 3, 4, 5 , Shuai Zhang 1, 2, 3, 4, 5 , Dewang Zeng 1, 2, 3, 4, 5 , Rui Xiao 1, 2, 3, 4, 5
Affiliation
|
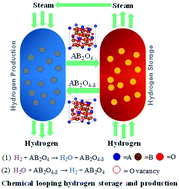
Chemical looping hydrogen storage and the recovery of iron oxides by the redox cycles were recommended as an emerging approach for large-scale hydrogen storage with a high volumetric hydrogen storage density. However, iron oxides should be operated at a high temperature (>800 °C) for its sufficient redox activity, which would lead to a rapid deterioration of hydrogen storage performance over cycles. In this work, a series of ferrite-spinel materials A0.25Fe2.75O4 (A = Co, Cu, Ni, Zn or Mn) were prepared. Among all the additives to iron oxides, Co0.25Fe2.75O4 exhibits the highest volumetric hydrogen storage density (∼62.47 g L−1) and an average hydrogen production rate (∼132 μmol g−1 min−1) under 550 °C. Besides, the storage capacity was maintained over 10 cycles. The volumetric hydrogen storage density of this material was proportionate to the most advanced Rh–FeOx containing rare-earth metal; thus, it may have the potential for industrial application.
中文翻译:

化学循环氢气的存储和生产:使用二元铁氧体-尖晶石作为氧气载体材料
推荐使用化学循环储氢和通过氧化还原循环回收氧化铁作为新兴的大规模储氢和高储氢密度的方法。但是,氧化铁应具有足够的氧化还原活性,应在高温(> 800°C)下运行,这会导致氢存储性能随周期迅速下降。在这项工作中,准备了一系列铁氧体尖晶石材料A 0.25 Fe 2.75 O 4(A = Co,Cu,Ni,Zn或Mn)。在所有氧化铁添加剂中,Co 0.25 Fe 2.75 O 4的储氢密度最高(〜62.47 g L -1)和在550°C下的平均产氢率(〜132μmolg -1 min -1)。此外,存储容量保持超过10个周期。这种材料的储氢体积密度与最高级的含Rh-FeO x的稀土金属成正比。因此,它可能具有工业应用的潜力。
更新日期:2020-03-31
中文翻译:

化学循环氢气的存储和生产:使用二元铁氧体-尖晶石作为氧气载体材料
推荐使用化学循环储氢和通过氧化还原循环回收氧化铁作为新兴的大规模储氢和高储氢密度的方法。但是,氧化铁应具有足够的氧化还原活性,应在高温(> 800°C)下运行,这会导致氢存储性能随周期迅速下降。在这项工作中,准备了一系列铁氧体尖晶石材料A 0.25 Fe 2.75 O 4(A = Co,Cu,Ni,Zn或Mn)。在所有氧化铁添加剂中,Co 0.25 Fe 2.75 O 4的储氢密度最高(〜62.47 g L -1)和在550°C下的平均产氢率(〜132μmolg -1 min -1)。此外,存储容量保持超过10个周期。这种材料的储氢体积密度与最高级的含Rh-FeO x的稀土金属成正比。因此,它可能具有工业应用的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号