当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The adsorption and growth of Agn (n = 1–4) clusters on cubic, monoclinic, and tetragonal ZrO2 surfaces: a first-principles study
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020/01/13 , DOI: 10.1039/c9nj03769a Tingting Liu 1, 2, 3, 4 , Yan Li 4, 5, 6, 7 , Changhai Liang 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020/01/13 , DOI: 10.1039/c9nj03769a Tingting Liu 1, 2, 3, 4 , Yan Li 4, 5, 6, 7 , Changhai Liang 1, 2, 3, 4
Affiliation
|
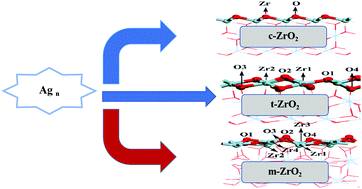
Supported metal nanoparticles are widely used as catalysts in the industrial production of chemicals, but still suffer from deactivation because of metal leaching and sintering at high temperature. In this work, the adsorption and growth of Agn (n = 1–4) on cubic zirconia (c-ZrO2) (1 1 1), monoclinic zirconia (m-ZrO2) (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1), and tetragonal zirconia (t-ZrO2) (1 0 1) surfaces has been examined with the aid of first-principles calculations and the periodic supercell model. The results show that the adsorption of a single silver atom on different zirconia surfaces follows the trend t-ZrO2(1 0 1) > m-ZrO2(
1 1), and tetragonal zirconia (t-ZrO2) (1 0 1) surfaces has been examined with the aid of first-principles calculations and the periodic supercell model. The results show that the adsorption of a single silver atom on different zirconia surfaces follows the trend t-ZrO2(1 0 1) > m-ZrO2(![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1) > c-ZrO2(1 1 1). The growth of Agn (n = 2–4) on different zirconia surfaces is thermodynamically feasible and the supports can improve the dispersion of the silver particles, especially the surface of t-ZrO2(1 0 1). Bader charge analysis was conducted on the most stable adsorption structure of the different zirconia surfaces for a single silver atom. The results show that no major charge transfer was observed when the silver atoms adsorbed on the stoichiometric c-ZrO2(1 1 1), t-ZrO2(1 0 1) and m-ZrO2(
1 1) > c-ZrO2(1 1 1). The growth of Agn (n = 2–4) on different zirconia surfaces is thermodynamically feasible and the supports can improve the dispersion of the silver particles, especially the surface of t-ZrO2(1 0 1). Bader charge analysis was conducted on the most stable adsorption structure of the different zirconia surfaces for a single silver atom. The results show that no major charge transfer was observed when the silver atoms adsorbed on the stoichiometric c-ZrO2(1 1 1), t-ZrO2(1 0 1) and m-ZrO2(![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1) surfaces, and the silver's unpaired electron remains largely localized on the metal adatom upon adsorption. These findings could help in understanding the metal cluster–support interactions and designing new catalysts for this kind of reaction.
1 1) surfaces, and the silver's unpaired electron remains largely localized on the metal adatom upon adsorption. These findings could help in understanding the metal cluster–support interactions and designing new catalysts for this kind of reaction.
中文翻译:

立方,单斜晶和四方ZrO2表面上Agn(n = 1-4)团簇的吸附和生长:一项第一性原理研究
负载的金属纳米颗粒在化学工业生产中被广泛用作催化剂,但由于金属的浸出和高温烧结而仍然失活。在这项工作中,Ag n(n = 1-4)在立方氧化锆(c-ZrO 2)(1 1 1),单斜氧化锆(m-ZrO 2)(![[1个结合宏]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1)和四方氧化锆(借助第一性原理计算和周期超单元模型检查了t-ZrO 2)(1 0 1)表面。结果表明,单个银原子在不同氧化锆表面上的吸附遵循t-ZrO 2(1 0 1)> m-ZrO 2(
1 1)和四方氧化锆(借助第一性原理计算和周期超单元模型检查了t-ZrO 2)(1 0 1)表面。结果表明,单个银原子在不同氧化锆表面上的吸附遵循t-ZrO 2(1 0 1)> m-ZrO 2(![[1个结合宏]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1)> c-ZrO 2趋势。(1 1 1)。Ag n(n = 2-4)在不同氧化锆表面上的生长在热力学上是可行的,并且载体可以改善银颗粒的分散,尤其是t-ZrO 2(1 0 1)的表面。对于单个银原子,对不同氧化锆表面的最稳定吸附结构进行了差电荷分析。结果表明,当银原子吸附在化学计量的c-ZrO 2(1 1 1),t-ZrO 2(1 0 1)和m-ZrO 2(
1 1)> c-ZrO 2趋势。(1 1 1)。Ag n(n = 2-4)在不同氧化锆表面上的生长在热力学上是可行的,并且载体可以改善银颗粒的分散,尤其是t-ZrO 2(1 0 1)的表面。对于单个银原子,对不同氧化锆表面的最稳定吸附结构进行了差电荷分析。结果表明,当银原子吸附在化学计量的c-ZrO 2(1 1 1),t-ZrO 2(1 0 1)和m-ZrO 2(![[1个结合宏]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1)表面,并且银的未成对电子在吸附后大部分保留在金属原子上。这些发现可能有助于理解金属簇与载体之间的相互作用,并为这种反应设计新的催化剂。
1 1)表面,并且银的未成对电子在吸附后大部分保留在金属原子上。这些发现可能有助于理解金属簇与载体之间的相互作用,并为这种反应设计新的催化剂。
更新日期:2020-02-13
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1), and tetragonal zirconia (t-ZrO2) (1 0 1) surfaces has been examined with the aid of first-principles calculations and the periodic supercell model. The results show that the adsorption of a single silver atom on different zirconia surfaces follows the trend t-ZrO2(1 0 1) > m-ZrO2(
1 1), and tetragonal zirconia (t-ZrO2) (1 0 1) surfaces has been examined with the aid of first-principles calculations and the periodic supercell model. The results show that the adsorption of a single silver atom on different zirconia surfaces follows the trend t-ZrO2(1 0 1) > m-ZrO2(![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1) > c-ZrO2(1 1 1). The growth of Agn (n = 2–4) on different zirconia surfaces is thermodynamically feasible and the supports can improve the dispersion of the silver particles, especially the surface of t-ZrO2(1 0 1). Bader charge analysis was conducted on the most stable adsorption structure of the different zirconia surfaces for a single silver atom. The results show that no major charge transfer was observed when the silver atoms adsorbed on the stoichiometric c-ZrO2(1 1 1), t-ZrO2(1 0 1) and m-ZrO2(
1 1) > c-ZrO2(1 1 1). The growth of Agn (n = 2–4) on different zirconia surfaces is thermodynamically feasible and the supports can improve the dispersion of the silver particles, especially the surface of t-ZrO2(1 0 1). Bader charge analysis was conducted on the most stable adsorption structure of the different zirconia surfaces for a single silver atom. The results show that no major charge transfer was observed when the silver atoms adsorbed on the stoichiometric c-ZrO2(1 1 1), t-ZrO2(1 0 1) and m-ZrO2(![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1) surfaces, and the silver's unpaired electron remains largely localized on the metal adatom upon adsorption. These findings could help in understanding the metal cluster–support interactions and designing new catalysts for this kind of reaction.
1 1) surfaces, and the silver's unpaired electron remains largely localized on the metal adatom upon adsorption. These findings could help in understanding the metal cluster–support interactions and designing new catalysts for this kind of reaction.
中文翻译:

立方,单斜晶和四方ZrO2表面上Agn(n = 1-4)团簇的吸附和生长:一项第一性原理研究
负载的金属纳米颗粒在化学工业生产中被广泛用作催化剂,但由于金属的浸出和高温烧结而仍然失活。在这项工作中,Ag n(n = 1-4)在立方氧化锆(c-ZrO 2)(1 1 1),单斜氧化锆(m-ZrO 2)(
![[1个结合宏]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1)和四方氧化锆(借助第一性原理计算和周期超单元模型检查了t-ZrO 2)(1 0 1)表面。结果表明,单个银原子在不同氧化锆表面上的吸附遵循t-ZrO 2(1 0 1)> m-ZrO 2(
1 1)和四方氧化锆(借助第一性原理计算和周期超单元模型检查了t-ZrO 2)(1 0 1)表面。结果表明,单个银原子在不同氧化锆表面上的吸附遵循t-ZrO 2(1 0 1)> m-ZrO 2(![[1个结合宏]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1)> c-ZrO 2趋势。(1 1 1)。Ag n(n = 2-4)在不同氧化锆表面上的生长在热力学上是可行的,并且载体可以改善银颗粒的分散,尤其是t-ZrO 2(1 0 1)的表面。对于单个银原子,对不同氧化锆表面的最稳定吸附结构进行了差电荷分析。结果表明,当银原子吸附在化学计量的c-ZrO 2(1 1 1),t-ZrO 2(1 0 1)和m-ZrO 2(
1 1)> c-ZrO 2趋势。(1 1 1)。Ag n(n = 2-4)在不同氧化锆表面上的生长在热力学上是可行的,并且载体可以改善银颗粒的分散,尤其是t-ZrO 2(1 0 1)的表面。对于单个银原子,对不同氧化锆表面的最稳定吸附结构进行了差电荷分析。结果表明,当银原子吸附在化学计量的c-ZrO 2(1 1 1),t-ZrO 2(1 0 1)和m-ZrO 2(![[1个结合宏]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1 1)表面,并且银的未成对电子在吸附后大部分保留在金属原子上。这些发现可能有助于理解金属簇与载体之间的相互作用,并为这种反应设计新的催化剂。
1 1)表面,并且银的未成对电子在吸附后大部分保留在金属原子上。这些发现可能有助于理解金属簇与载体之间的相互作用,并为这种反应设计新的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号