当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Specific Cleavage of an RNA Repeat Expansion with a Dimeric Small Molecule Is Advantageous over Sequence-Specific Recognition by an Oligonucleotide.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-13 , DOI: 10.1021/acschembio.9b00958 Raphael I Benhamou 1 , Alicia J Angelbello 1 , Ryan J Andrews 2 , Eric T Wang 3 , Walter N Moss 2 , Matthew D Disney 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-13 , DOI: 10.1021/acschembio.9b00958 Raphael I Benhamou 1 , Alicia J Angelbello 1 , Ryan J Andrews 2 , Eric T Wang 3 , Walter N Moss 2 , Matthew D Disney 1
Affiliation
|
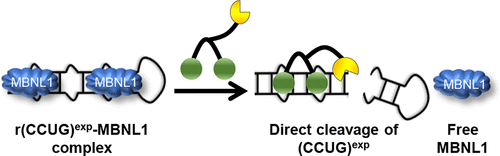
Myotonic dystrophy type 2 (DM2) is a genetically defined muscular dystrophy that is caused by an expanded repeat of r(CCUG) [r(CCUG)exp] in intron 1 of a CHC-type zinc finger nucleic acid binding protein (CNBP) pre-mRNA. Various mechanisms contribute to DM2 pathology including pre-mRNA splicing defects caused by sequestration of the RNA splicing regulator muscleblind-like-1 (MBNL1) by r(CCUG)exp. Herein, we study the biological impacts of the molecular recognition of r(CCUG)exp's structure by a designer dimeric small molecule that directly cleaves the RNA in patient-derived cells. The compound is comprised of two RNA-binding modules conjugated to a derivative of the natural product bleomycin. Careful design of the chimera affords RNA-specific cleavage, as attachment of the bleomycin cleaving module was done in a manner that disables DNA cleavage. The chimeric cleaver is more potent than the parent binding compound for alleviating DM2-associated defects. Importantly, oligonucleotides targeting the r(CCUG)exp sequence for cleavage exacerbate DM2 defects due to recognition of a short r(CCUG) sequence that is embedded in CNBP, argonaute-1 (AGO1), and MBNL1, reducing their levels. The latter event causes a greater depletion of functional MBNL1 than the amount already sequestered by r(CCUG)exp. Thus, compounds targeting RNA structures can have functional advantages over oligonucleotides that target the sequence in some disease settings, particularly in DM2.
中文翻译:

使用二聚体小分子对 RNA 重复扩增进行结构特异性切割优于寡核苷酸的序列特异性识别。
强直性肌营养不良 2 型 (DM2) 是一种基因定义的肌营养不良症,由 CHC 型锌指核酸结合蛋白 (CNBP) 前内含子 1 中 r(CCUG) [r(CCUG)exp] 的重复扩增引起。 -mRNA。多种机制导致 DM2 病理,包括 r(CCUG)exp 隔离 RNA 剪接调节因子muscleblind-like-1 (MBNL1) 引起的前 mRNA 剪接缺陷。在此,我们研究了设计的二聚小分子对 r(CCUG)exp 结构的分子识别的生物学影响,该小分子直接切割患者来源细胞中的 RNA。该化合物由两个与天然产物博莱霉素衍生物缀合的 RNA 结合模块组成。嵌合体的精心设计提供了 RNA 特异性切割,因为博莱霉素切割模块的连接是以禁用 DNA 切割的方式进行的。嵌合切割器比母体结合化合物更有效地减轻 DM2 相关缺陷。重要的是,针对 r(CCUG)exp 序列进行切割的寡核苷酸会加剧 DM2 缺陷,这是由于识别了嵌入 CNBP、argonaute-1 (AGO1) 和 MBNL1 中的短 r(CCUG) 序列,从而降低了它们的水平。后一事件导致功能性 MBNL1 的消耗比 r(CCUG)exp 已经隔离的量更大。因此,在某些疾病环境中,特别是在 DM2 中,靶向 RNA 结构的化合物比靶向该序列的寡核苷酸具有功能优势。
更新日期:2020-01-13
中文翻译:

使用二聚体小分子对 RNA 重复扩增进行结构特异性切割优于寡核苷酸的序列特异性识别。
强直性肌营养不良 2 型 (DM2) 是一种基因定义的肌营养不良症,由 CHC 型锌指核酸结合蛋白 (CNBP) 前内含子 1 中 r(CCUG) [r(CCUG)exp] 的重复扩增引起。 -mRNA。多种机制导致 DM2 病理,包括 r(CCUG)exp 隔离 RNA 剪接调节因子muscleblind-like-1 (MBNL1) 引起的前 mRNA 剪接缺陷。在此,我们研究了设计的二聚小分子对 r(CCUG)exp 结构的分子识别的生物学影响,该小分子直接切割患者来源细胞中的 RNA。该化合物由两个与天然产物博莱霉素衍生物缀合的 RNA 结合模块组成。嵌合体的精心设计提供了 RNA 特异性切割,因为博莱霉素切割模块的连接是以禁用 DNA 切割的方式进行的。嵌合切割器比母体结合化合物更有效地减轻 DM2 相关缺陷。重要的是,针对 r(CCUG)exp 序列进行切割的寡核苷酸会加剧 DM2 缺陷,这是由于识别了嵌入 CNBP、argonaute-1 (AGO1) 和 MBNL1 中的短 r(CCUG) 序列,从而降低了它们的水平。后一事件导致功能性 MBNL1 的消耗比 r(CCUG)exp 已经隔离的量更大。因此,在某些疾病环境中,特别是在 DM2 中,靶向 RNA 结构的化合物比靶向该序列的寡核苷酸具有功能优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号