当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantifying the insertion of membrane proteins into lipid bilayer nanodiscs using a fusion protein strategy.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.bbamem.2020.183190 Elisabeth Häusler 1 , Kai Fredriksson 1 , Inguna Goba 1 , Carsten Peters 2 , Kolio Raltchev 1 , Laura Sperl 1 , Andrea Steiner 1 , Sevil Weinkauf 2 , Franz Hagn 1
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.bbamem.2020.183190 Elisabeth Häusler 1 , Kai Fredriksson 1 , Inguna Goba 1 , Carsten Peters 2 , Kolio Raltchev 1 , Laura Sperl 1 , Andrea Steiner 1 , Sevil Weinkauf 2 , Franz Hagn 1
Affiliation
|
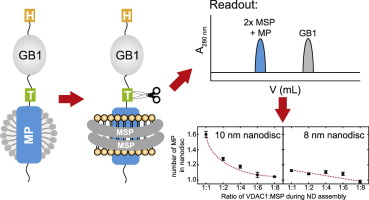
A membrane protein's oligomeric state modulates its functionality in various cellular processes. Since membrane proteins have to be solubilized in an appropriate membrane mimetic, the use of classical biophysical methods to analyze protein oligomers is challenging. We here present a method to determine the number of membrane proteins inserted into lipid nanodiscs. It is based on the ability to selectively quantify the amount of a small and robust fusion protein that can be proteolytically cleaved off from a membrane protein after incorporation into lipid nanodiscs. A detailed knowledge of the number of membrane proteins per nanodisc at defined assembly conditions is essential to estimate the tendency for oligomerization, but also for guiding sample optimization for structural investigations that require the presence of a homogenous oligomeric state. We show that this method can efficiently be used to determine the number of VDAC1 channels in nanodiscs at various assembly conditions, as confirmed by negative stain EM. The presented method is suitable in particular for membrane proteins that cannot be probed easily by other methods such as single span transmembrane helices. This assay can be applied to any membrane protein that can be incorporated into a nanodisc without the requirement for special instrumentation and will thus be widely applicable and complementary to other methods that quantify membrane protein insertion in lipid nanodiscs.
中文翻译:

使用融合蛋白策略定量将膜蛋白插入脂质双层纳米光盘中。
膜蛋白的低聚状态会在各种细胞过程中调节其功能。由于必须将膜蛋白溶解在合适的膜模拟物中,因此使用经典的生物物理方法来分析蛋白低聚物具有挑战性。我们在这里提出一种确定插入脂质纳米光盘的膜蛋白数量的方法。它基于选择性定量小的坚固的融合蛋白的量的能力,这种融合蛋白在掺入脂质纳米盘后可以从膜蛋白上进行蛋白水解切割。在定义的组装条件下,每个纳米光盘的膜蛋白数量的详细知识对于估算寡聚化趋势至关重要,还可以指导样品优化,以进行需要均质低聚状态的结构研究。我们表明,该方法可以有效地用于确定各种组装条件下纳米光盘中VDAC1通道的数量,这已由EM负染色证实。提出的方法特别适用于不能通过其他方法(例如单跨跨膜螺旋)轻易探测的膜蛋白。该测定法可应用于无需特殊仪器即可掺入纳米圆盘的任何膜蛋白,因此将广泛适用,并与定量脂质纳米圆盘中膜蛋白插入的其他方法互补。我们表明,该方法可以有效地用于确定各种组装条件下纳米光盘中VDAC1通道的数量,这已由EM负染色证实。提出的方法特别适用于不能通过其他方法(例如单跨跨膜螺旋)轻易探测的膜蛋白。该测定法可应用于无需特殊仪器即可掺入纳米圆盘的任何膜蛋白,因此将广泛适用,并与定量脂质纳米圆盘中膜蛋白插入的其他方法互补。我们表明,该方法可以有效地用于确定各种组装条件下纳米光盘中VDAC1通道的数量,这已被EM阴性染色所证实。提出的方法特别适用于不能通过其他方法(例如单跨跨膜螺旋)轻易探测的膜蛋白。该测定法可应用于无需特殊仪器即可掺入纳米光盘中的任何膜蛋白,因此将广泛适用,并与定量脂质纳米盘中膜蛋白插入的其他方法互补。
更新日期:2020-01-13
中文翻译:

使用融合蛋白策略定量将膜蛋白插入脂质双层纳米光盘中。
膜蛋白的低聚状态会在各种细胞过程中调节其功能。由于必须将膜蛋白溶解在合适的膜模拟物中,因此使用经典的生物物理方法来分析蛋白低聚物具有挑战性。我们在这里提出一种确定插入脂质纳米光盘的膜蛋白数量的方法。它基于选择性定量小的坚固的融合蛋白的量的能力,这种融合蛋白在掺入脂质纳米盘后可以从膜蛋白上进行蛋白水解切割。在定义的组装条件下,每个纳米光盘的膜蛋白数量的详细知识对于估算寡聚化趋势至关重要,还可以指导样品优化,以进行需要均质低聚状态的结构研究。我们表明,该方法可以有效地用于确定各种组装条件下纳米光盘中VDAC1通道的数量,这已由EM负染色证实。提出的方法特别适用于不能通过其他方法(例如单跨跨膜螺旋)轻易探测的膜蛋白。该测定法可应用于无需特殊仪器即可掺入纳米圆盘的任何膜蛋白,因此将广泛适用,并与定量脂质纳米圆盘中膜蛋白插入的其他方法互补。我们表明,该方法可以有效地用于确定各种组装条件下纳米光盘中VDAC1通道的数量,这已由EM负染色证实。提出的方法特别适用于不能通过其他方法(例如单跨跨膜螺旋)轻易探测的膜蛋白。该测定法可应用于无需特殊仪器即可掺入纳米圆盘的任何膜蛋白,因此将广泛适用,并与定量脂质纳米圆盘中膜蛋白插入的其他方法互补。我们表明,该方法可以有效地用于确定各种组装条件下纳米光盘中VDAC1通道的数量,这已被EM阴性染色所证实。提出的方法特别适用于不能通过其他方法(例如单跨跨膜螺旋)轻易探测的膜蛋白。该测定法可应用于无需特殊仪器即可掺入纳米光盘中的任何膜蛋白,因此将广泛适用,并与定量脂质纳米盘中膜蛋白插入的其他方法互补。











































 京公网安备 11010802027423号
京公网安备 11010802027423号