当前位置:
X-MOL 学术
›
BBA Bioenerg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding of red form of Orange Carotenoid Protein (OCP) to phycobilisome is not sufficient for quenching.
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.bbabio.2020.148155 Wenjing Lou 1 , Dariusz M Niedzwiedzki 2 , Ruidong J Jiang 3 , Robert E Blankenship 4 , Haijun Liu 4
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.bbabio.2020.148155 Wenjing Lou 1 , Dariusz M Niedzwiedzki 2 , Ruidong J Jiang 3 , Robert E Blankenship 4 , Haijun Liu 4
Affiliation
|
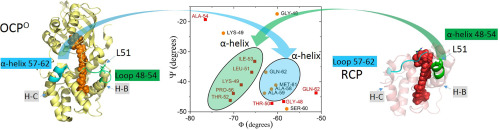
The Orange Carotenoid Protein (OCP) is responsible for photoprotection in many cyanobacteria. Absorption of blue light drives the conversion of the orange, inactive form (OCPO) to the red, active form (OCPR). Concomitantly, the N-terminal domain (NTD) and the C-terminal domain (CTD) of OCP separate, which ultimately leads to the formation of a quenched OCPR-PBS complex. The details of the photoactivation of OCP have been intensely researched. Binding site(s) of OCPR on the PBS core have also been proposed. However, the post-binding events of the OCPR-PBS complex remain unclear. Here, we demonstrate that PBS-bound OCPR is not sufficient as a PBS excitation energy quencher. Using site-directed mutagenesis, we generated a suite of single point mutations at OCP Leucine 51 (L51) of Synechocystis 6803. Steady-state and time-resolved fluorescence analyses demonstrated that all mutant proteins are unable to quench the PBS fluorescence, owing to either failed OCP binding to PBS, or, if bound, an OCP-PBS quenching state failed to form. The SDS-PAGE and Western blot analysis support that the L51A (Alanine) mutant binds to the PBS and therefore belongs to the second category. We hypothesize that upon binding to PBS, OCPR likely reorganizes and adopts a new conformational state (OCP3rd) different than either OCPO or OCPR to allow energy quenching, depending on the cross-talk between OCPR and its PBS core-binding counterpart.
中文翻译:

红色形式的橙色类胡萝卜素蛋白(OCP)与藻胆体的结合不足以淬灭。
橙色类胡萝卜素蛋白(OCP)负责许多蓝细菌的光保护。蓝光的吸收驱动橙色非活性形式(OCPO)转换为红色活性形式(OCPR)。同时,OCP的N末端结构域(NTD)和C末端结构域(CTD)分开,最终导致淬灭的OCPR-PBS复合物形成。对OCP的光活化的细节已进行了深入研究。还提出了OCPR在PBS核心上的结合位点。但是,OCPR-PBS复合物的结合后事件仍然不清楚。在这里,我们证明了PBS结合的OCPR不足以作为PBS激发能猝灭剂。使用定点诱变,我们在集胞藻6803的OCP亮氨酸51(L51)处产生了一组单点突变。稳态和时间分辨荧光分析表明,由于OCP与PBS结合失败,或者如果形成,则无法形成OCP-PBS淬灭状态,因此所有突变蛋白均无法淬灭PBS荧光。SDS-PAGE和蛋白质印迹分析支持L51A(丙氨酸)突变体与PBS结合,因此属于第二类。我们假设与PBS结合后,OCPR可能会重组并采用不同于OCPO或OCPR的新构象状态(OCP3rd)以允许能量猝灭,这取决于OCPR及其与PBS核心结合的对应物之间的串扰。SDS-PAGE和蛋白质印迹分析支持L51A(丙氨酸)突变体与PBS结合,因此属于第二类。我们假设与PBS结合后,OCPR可能会重组并采用不同于OCPO或OCPR的新构象状态(OCP3rd)以允许能量猝灭,这取决于OCPR及其与PBS核心结合的对应物之间的串扰。SDS-PAGE和蛋白质印迹分析支持L51A(丙氨酸)突变体与PBS结合,因此属于第二类。我们假设与PBS结合后,OCPR可能会重组并采用不同于OCPO或OCPR的新构象状态(OCP3rd)以允许能量猝灭,这取决于OCPR及其与PBS核心结合的对应物之间的串扰。
更新日期:2020-01-13
中文翻译:

红色形式的橙色类胡萝卜素蛋白(OCP)与藻胆体的结合不足以淬灭。
橙色类胡萝卜素蛋白(OCP)负责许多蓝细菌的光保护。蓝光的吸收驱动橙色非活性形式(OCPO)转换为红色活性形式(OCPR)。同时,OCP的N末端结构域(NTD)和C末端结构域(CTD)分开,最终导致淬灭的OCPR-PBS复合物形成。对OCP的光活化的细节已进行了深入研究。还提出了OCPR在PBS核心上的结合位点。但是,OCPR-PBS复合物的结合后事件仍然不清楚。在这里,我们证明了PBS结合的OCPR不足以作为PBS激发能猝灭剂。使用定点诱变,我们在集胞藻6803的OCP亮氨酸51(L51)处产生了一组单点突变。稳态和时间分辨荧光分析表明,由于OCP与PBS结合失败,或者如果形成,则无法形成OCP-PBS淬灭状态,因此所有突变蛋白均无法淬灭PBS荧光。SDS-PAGE和蛋白质印迹分析支持L51A(丙氨酸)突变体与PBS结合,因此属于第二类。我们假设与PBS结合后,OCPR可能会重组并采用不同于OCPO或OCPR的新构象状态(OCP3rd)以允许能量猝灭,这取决于OCPR及其与PBS核心结合的对应物之间的串扰。SDS-PAGE和蛋白质印迹分析支持L51A(丙氨酸)突变体与PBS结合,因此属于第二类。我们假设与PBS结合后,OCPR可能会重组并采用不同于OCPO或OCPR的新构象状态(OCP3rd)以允许能量猝灭,这取决于OCPR及其与PBS核心结合的对应物之间的串扰。SDS-PAGE和蛋白质印迹分析支持L51A(丙氨酸)突变体与PBS结合,因此属于第二类。我们假设与PBS结合后,OCPR可能会重组并采用不同于OCPO或OCPR的新构象状态(OCP3rd)以允许能量猝灭,这取决于OCPR及其与PBS核心结合的对应物之间的串扰。











































 京公网安备 11010802027423号
京公网安备 11010802027423号