当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Epigenetic Silencing of CDR1as Drives IGF2BP3-Mediated Melanoma Invasion and Metastasis.
Cancer Cell ( IF 48.8 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.ccell.2019.12.007 Douglas Hanniford 1 , Alejandro Ulloa-Morales 1 , Alcida Karz 1 , Maria Gabriela Berzoti-Coelho 2 , Rana S Moubarak 1 , Beatriz Sánchez-Sendra 3 , Andreas Kloetgen 1 , Veronica Davalos 4 , Jochen Imig 1 , Pamela Wu 5 , Varshini Vasudevaraja 6 , Diana Argibay 1 , Karin Lilja 7 , Tommaso Tabaglio 8 , Carlos Monteagudo 9 , Ernesto Guccione 10 , Aristotelis Tsirigos 11 , Iman Osman 12 , Iannis Aifantis 1 , Eva Hernando 1
Cancer Cell ( IF 48.8 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.ccell.2019.12.007 Douglas Hanniford 1 , Alejandro Ulloa-Morales 1 , Alcida Karz 1 , Maria Gabriela Berzoti-Coelho 2 , Rana S Moubarak 1 , Beatriz Sánchez-Sendra 3 , Andreas Kloetgen 1 , Veronica Davalos 4 , Jochen Imig 1 , Pamela Wu 5 , Varshini Vasudevaraja 6 , Diana Argibay 1 , Karin Lilja 7 , Tommaso Tabaglio 8 , Carlos Monteagudo 9 , Ernesto Guccione 10 , Aristotelis Tsirigos 11 , Iman Osman 12 , Iannis Aifantis 1 , Eva Hernando 1
Affiliation
|
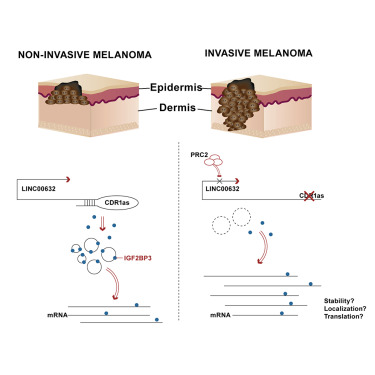
Metastasis is the primary cause of death of cancer patients. Dissecting mechanisms governing metastatic spread may uncover important tumor biology and/or yield promising therapeutic insights. Here, we investigated the role of circular RNAs (circRNA) in metastasis, using melanoma as a model aggressive tumor. We identified silencing of cerebellar degeneration-related 1 antisense (CDR1as), a regulator of miR-7, as a hallmark of melanoma progression. CDR1as depletion results from epigenetic silencing of LINC00632, its originating long non-coding RNA (lncRNA) and promotes invasion in vitro and metastasis in vivo through a miR-7-independent, IGF2BP3-mediated mechanism. Moreover, CDR1as levels reflect cellular states associated with distinct therapeutic responses. Our study reveals functional, prognostic, and predictive roles for CDR1as and expose circRNAs as key players in metastasis.
中文翻译:

CDR1as 的表观遗传沉默驱动 IGF2BP3 介导的黑色素瘤侵袭和转移。
转移是癌症患者死亡的主要原因。剖析控制转移扩散的机制可能会揭示重要的肿瘤生物学和/或产生有希望的治疗见解。在这里,我们使用黑色素瘤作为侵袭性肿瘤模型,研究了环状 RNA (circRNA) 在转移中的作用。我们发现小脑变性相关 1 反义基因 (CDR1as)(miR-7 的调节因子)的沉默是黑色素瘤进展的标志。 CDR1as 耗竭是由 LINC00632(其起源的长非编码 RNA (lncRNA))表观遗传沉默引起的,并通过不依赖 miR-7 的 IGF2BP3 介导的机制促进体外侵袭和体内转移。此外,CDR1as 水平反映了与不同治疗反应相关的细胞状态。我们的研究揭示了 CDR1as 的功能、预后和预测作用,并揭示了 circRNA 在转移中的关键作用。
更新日期:2020-01-13
中文翻译:

CDR1as 的表观遗传沉默驱动 IGF2BP3 介导的黑色素瘤侵袭和转移。
转移是癌症患者死亡的主要原因。剖析控制转移扩散的机制可能会揭示重要的肿瘤生物学和/或产生有希望的治疗见解。在这里,我们使用黑色素瘤作为侵袭性肿瘤模型,研究了环状 RNA (circRNA) 在转移中的作用。我们发现小脑变性相关 1 反义基因 (CDR1as)(miR-7 的调节因子)的沉默是黑色素瘤进展的标志。 CDR1as 耗竭是由 LINC00632(其起源的长非编码 RNA (lncRNA))表观遗传沉默引起的,并通过不依赖 miR-7 的 IGF2BP3 介导的机制促进体外侵袭和体内转移。此外,CDR1as 水平反映了与不同治疗反应相关的细胞状态。我们的研究揭示了 CDR1as 的功能、预后和预测作用,并揭示了 circRNA 在转移中的关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号