当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relationship among structure, cytotoxicity, and Michael acceptor reactivity of quinocidin.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.bmc.2020.115308 Yu Nakagawa 1 , Yuki Sawaki 1 , Wataru Miyanishi 1 , Sayako Shimomura 1 , Takahiro Shibata 1 , Makoto Ojika 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.bmc.2020.115308 Yu Nakagawa 1 , Yuki Sawaki 1 , Wataru Miyanishi 1 , Sayako Shimomura 1 , Takahiro Shibata 1 , Makoto Ojika 1
Affiliation
|
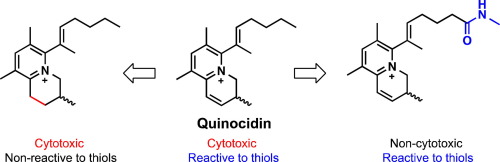
Quinocidin (QCD) is a cytotoxic antibiotic with an unusual 3,4-dihydroquinolizinium skeleton. We previously found that QCD captures thiols in neutral aqueous media via a Michael addition-type reaction. However, it remains unclear whether the Michael acceptor reactivity of QCD is responsible for its cytotoxicity. In this study, we synthesized thirteen analogs of QCD to examine the relationship among its structure, cytotoxicity, and reactivity toward thiols. Thiol-trapping experiments and cytotoxicity tests collectively suggested that the Michael acceptor function of QCD is independent of its cytotoxic activity, and that the pyridinium moiety with the hydrophobic side chain is a key structural factor for cytotoxicity. These findings further led us to demonstrate that incorporation of an amide group into the side chain of QCD significantly reduced its toxicity but hardly affected the Michael acceptor function. The present study lays the foundation for QCD-based drug design and highlights the potential of QCD as a unique electrophile for use in the development of covalent inhibitors and protein-labeling probes.
中文翻译:

喹诺丁的结构,细胞毒性和迈克尔受体反应性之间的关系。
奎尼丁(QCD)是具有非常规3,4-二氢喹啉鎓骨架的细胞毒性抗生素。我们先前发现QCD通过迈克尔加成型反应在中性水性介质中捕获硫醇。但是,尚不清楚QCD的Michael受体反应性是否对其细胞毒性负责。在这项研究中,我们合成了QCD的13种类似物,以研究其结构,细胞毒性和对硫醇的反应性之间的关系。硫醇捕获实验和细胞毒性试验共同表明,QCD的迈克尔受体功能与其细胞毒性活性无关,具有疏水侧链的吡啶鎓部分是细胞毒性的关键结构因素。这些发现进一步使我们证明,将酰胺基团掺入QCD的侧链可显着降低其毒性,但几乎不影响迈克尔受体的功能。本研究为基于QCD的药物设计奠定了基础,并强调了QCD作为独特的亲电子试剂用于共价抑制剂和蛋白质标记探针开发的潜力。
更新日期:2020-01-13
中文翻译:

喹诺丁的结构,细胞毒性和迈克尔受体反应性之间的关系。
奎尼丁(QCD)是具有非常规3,4-二氢喹啉鎓骨架的细胞毒性抗生素。我们先前发现QCD通过迈克尔加成型反应在中性水性介质中捕获硫醇。但是,尚不清楚QCD的Michael受体反应性是否对其细胞毒性负责。在这项研究中,我们合成了QCD的13种类似物,以研究其结构,细胞毒性和对硫醇的反应性之间的关系。硫醇捕获实验和细胞毒性试验共同表明,QCD的迈克尔受体功能与其细胞毒性活性无关,具有疏水侧链的吡啶鎓部分是细胞毒性的关键结构因素。这些发现进一步使我们证明,将酰胺基团掺入QCD的侧链可显着降低其毒性,但几乎不影响迈克尔受体的功能。本研究为基于QCD的药物设计奠定了基础,并强调了QCD作为独特的亲电子试剂用于共价抑制剂和蛋白质标记探针开发的潜力。










































 京公网安备 11010802027423号
京公网安备 11010802027423号