Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.tetlet.2020.151606 Raju Suresh Kumar , Abdulrahman I. Almansour , Natarajan Arumugam , Raju Ranjith Kumar
|
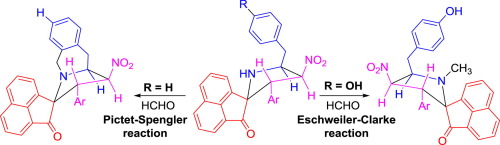
The 1,3-dipolar cycloaddition of azomethine ylides generated in situ from the decarboxylative condensation of acenaphthenequinone and tyrosine or phenylalanine to β-nitrostyrenes afforded spiro acenaphthylene-pyrrolidine hybrids. The subsequent reaction of these spiro heterocycles with formaldehyde and trifluoroacetic acid led to the formation of either Pictet-Spengler or Eschweiler-Clarke products depending on the substitution in the benzyl ring. The unsubstituted compounds occurred via Pictet-Spengler route to afford spiro acenaphthylene pyrrolo[1,2-b]isoquinoline hybrids as expected, whereas the 4-OH substituted compounds proceeded via Eschweiler-Clarke route affording the unexpected N-methylated spiro acenaphthylene-pyrrolidine hybrids.
中文翻译:

Pictet-Spengler和Eschweiler-Clarke反应之间的取代诱导转换:螺并吡咯并[1,2- b ]-异喹啉/吡咯烷杂化物的选择性合成
从啶醌和酪氨酸或苯丙氨酸的脱羧缩合到β-硝基苯乙烯的原位产生的偶氮甲碱的1,3-偶极环加成反应得到螺iro烯-吡咯烷杂化物。这些螺环杂环与甲醛和三氟乙酸的后续反应导致形成Pictet-Spengler或Eschweiler-Clarke产物,具体取决于苄环上的取代。未取代的化合物通过Pictet-Spengler途径出现,提供了预期的螺并吡咯并[1,2- b ]异喹啉杂化物,而4-OH取代的化合物通过Eschweiler-Clarke途径进行了处理,从而提供了出乎意料的N-甲基化的螺iro并吡咯烷-吡咯烷杂化物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号