当前位置:
X-MOL 学术
›
JAMA Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ambroxol for the Treatment of Patients With Parkinson Disease With and Without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial.
JAMA Neurology ( IF 20.4 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamaneurol.2019.4611 Stephen Mullin 1, 2 , Laura Smith 1 , Katherine Lee 1 , Gayle D'Souza 3 , Philip Woodgate 3 , Josh Elflein 3 , Jenny Hällqvist 4 , Marco Toffoli 1 , Adam Streeter 5 , Joanne Hosking 5 , Wendy E Heywood 4 , Rajeshree Khengar 3 , Philip Campbell 1 , Jason Hehir 6 , Sarah Cable 1 , Kevin Mills 4 , Henrik Zetterberg 7, 8, 9, 10 , Patricia Limousin 1 , Vincenzo Libri 3 , Tom Foltynie 1 , Anthony H V Schapira 1
JAMA Neurology ( IF 20.4 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamaneurol.2019.4611 Stephen Mullin 1, 2 , Laura Smith 1 , Katherine Lee 1 , Gayle D'Souza 3 , Philip Woodgate 3 , Josh Elflein 3 , Jenny Hällqvist 4 , Marco Toffoli 1 , Adam Streeter 5 , Joanne Hosking 5 , Wendy E Heywood 4 , Rajeshree Khengar 3 , Philip Campbell 1 , Jason Hehir 6 , Sarah Cable 1 , Kevin Mills 4 , Henrik Zetterberg 7, 8, 9, 10 , Patricia Limousin 1 , Vincenzo Libri 3 , Tom Foltynie 1 , Anthony H V Schapira 1
Affiliation
|
Importance
Mutations of the glucocerebrosidase gene, GBA1 (OMIM 606463), are the most important risk factor for Parkinson disease (PD). In vitro and in vivo studies have reported that ambroxol increases β-glucocerebrosidase (GCase) enzyme activity and reduces α-synuclein levels. These observations support a potential role for ambroxol therapy in modifying a relevant pathogenetic pathway in PD.
Objective
To assess safety, tolerability, cerebrospinal fluid (CSF) penetration, and target engagement of ambroxol therapy with GCase in patients with PD with and without GBA1 mutations.
Interventions
An escalating dose of oral ambroxol to 1.26 g per day.
Design, Setting, and Participants
This single-center open-label noncontrolled clinical trial was conducted between January 11, 2017, and April 25, 2018, at the Leonard Wolfson Experimental Neuroscience Centre, a dedicated clinical research facility and part of the University College London Queen Square Institute of Neurology in London, United Kingdom. Participants were recruited from established databases at the Royal Free London Hospital and National Hospital for Neurology and Neurosurgery in London. Twenty-four patients with moderate PD were evaluated for eligibility, and 23 entered the study. Of those, 18 patients completed the study; 1 patient was excluded (failed lumbar puncture), and 4 patients withdrew (predominantly lumbar puncture-related complications). All data analyses were performed from November 1 to December 14, 2018.
Main Outcomes and Measures
Primary outcomes at 186 days were the detection of ambroxol in the CSF and a change in CSF GCase activity.
Results
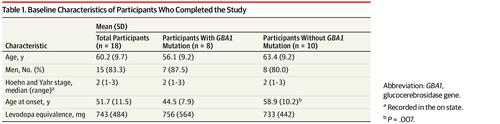
Of the 18 participants (15 men [83.3%]; mean [SD] age, 60.2 [9.7] years) who completed the study, 17 (8 with GBA1 mutations and 9 without GBA1 mutations) were included in the primary analysis. Between days 0 and 186, a 156-ng/mL increase in the level of ambroxol in CSF (lower 95% confidence limit, 129 ng/mL; P < .001) was observed. The CSF GCase activity decreased by 19% (0.059 nmol/mL per hour; 95% CI, -0.115 to -0.002; P = .04). The ambroxol therapy was well tolerated, with no serious adverse events. An increase of 50 pg/mL (13%) in the CSF α-synuclein concentration (95% CI, 14-87; P = .01) and an increase of 88 ng/mol (35%) in the CSF GCase protein levels (95% CI, 40-137; P = .002) were observed. Mean (SD) scores on part 3 of the Movement Disorders Society Unified Parkinson Disease Rating Scale decreased (ie, improved) by 6.8 (7.1) points (95% CI, -10.4 to -3.1; P = .001). These changes were observed in patients with and without GBA1 mutations.
Conclusions and Relevance
The study results suggest that ambroxol therapy was safe and well tolerated; CSF penetration and target engagement of ambroxol were achieved, and CSF α-synuclein levels were increased. Placebo-controlled clinical trials are needed to examine whether ambroxol therapy is associated with changes in the natural progression of PD.
Trial Registration
ClinicalTrials.gov identifier: NCT02941822; EudraCT identifier: 2015-002571-24.
中文翻译:

氨溴索治疗帕金森病患者是否患有葡萄糖脑苷脂酶基因突变:一项非随机,非对照试验。
重要性葡糖脑苷脂酶基因GBA1(OMIM 606463)的突变是帕金森病(PD)的最重要危险因素。体外和体内研究表明,氨溴索可增加β-葡萄糖脑苷脂酶(GCase)的酶活性并降低α-突触核蛋白的水平。这些观察结果支持氨溴索治疗可能会改变PD中的相关致病途径。目的评估具有或不具有GBA1突变的PD患者的安全性,耐受性,脑脊液(CSF)的渗透性以及使用GCase的氨溴索治疗的靶指标。干预措施口服氨溴索的剂量逐渐增加到每天1.26 g。设计,设置和参加者该单中心开放标签非对照临床试验于2017年1月11日至2018年4月25日进行,位于伦纳德·沃尔夫森(Leonard Wolfson)实验神经科学中心,这是一家专门的临床研究机构,是英国伦敦大学伦敦大学皇后广场神经学研究所的一部分。参与者是从伦敦皇家自由医院和伦敦国家神经病学与神经外科国家医院建立的数据库中招募的。对24例中度PD患者进行了资格评估,其中23例进入了研究。其中,有18名患者完成了研究。排除1例患者(腰椎穿刺失败),退出4例患者(主要是腰椎穿刺相关并发症)。所有数据分析均于2018年11月1日至12月14日进行。主要结果和措施186天的主要结果是脑脊液中氨溴索的检出和脑脊液GCase活性的变化。结果完成研究的18名参与者(15名男性[83.3%];平均[SD]年龄,60.2 [9.7]岁)中,有17名参与者(其中8名具有GBA1突变,9名没有GBA1突变)被包括在主要分析中。在第0天和第186天之间,观察到CSF中氨溴索水平增加156 ng / mL(较低的95%置信限,129 ng / mL; P <.001)。CSF GCase活性降低了19%(每小时0.059 nmol / mL; 95%CI,-0.115至-0.002; P = .04)。氨溴索疗法耐受良好,无严重不良事件。CSFα-突触核蛋白浓度增加50 pg / mL(13%)(95%CI,14-87; P = 0.01),CSF GCase蛋白水平增加88 ng / mol(35%)观察到(95%CI,40-137; P = .002)。运动障碍学会帕金森病统一评分量表第3部分的平均(SD)得分降低了6.8(7)。1)分(95%CI,-10.4至-3.1; P = 0.001)。在有和没有GBA1突变的患者中观察到了这些变化。结论与相关性研究结果表明氨溴索治疗是安全且耐受性良好的。实现了脑脊液渗透和氨溴索的靶定参与,并且脑脊液α-突触核蛋白水平增加。需要安慰剂对照的临床试验来检查氨溴索治疗是否与PD自然进程的改变有关。试验注册ClinicalTrials.gov标识符:NCT02941822;EudraCT标识符:2015-002571-24。和脑脊液α-突触核蛋白水平升高。需要安慰剂对照的临床试验来检查氨溴索治疗是否与PD自然进程的改变有关。试验注册ClinicalTrials.gov标识符:NCT02941822;EudraCT标识符:2015-002571-24。和脑脊液α-突触核蛋白水平升高。需要安慰剂对照的临床试验来检查氨溴索治疗是否与PD自然进程的改变有关。试验注册ClinicalTrials.gov标识符:NCT02941822;EudraCT标识符:2015-002571-24。
更新日期:2020-04-01
中文翻译:

氨溴索治疗帕金森病患者是否患有葡萄糖脑苷脂酶基因突变:一项非随机,非对照试验。
重要性葡糖脑苷脂酶基因GBA1(OMIM 606463)的突变是帕金森病(PD)的最重要危险因素。体外和体内研究表明,氨溴索可增加β-葡萄糖脑苷脂酶(GCase)的酶活性并降低α-突触核蛋白的水平。这些观察结果支持氨溴索治疗可能会改变PD中的相关致病途径。目的评估具有或不具有GBA1突变的PD患者的安全性,耐受性,脑脊液(CSF)的渗透性以及使用GCase的氨溴索治疗的靶指标。干预措施口服氨溴索的剂量逐渐增加到每天1.26 g。设计,设置和参加者该单中心开放标签非对照临床试验于2017年1月11日至2018年4月25日进行,位于伦纳德·沃尔夫森(Leonard Wolfson)实验神经科学中心,这是一家专门的临床研究机构,是英国伦敦大学伦敦大学皇后广场神经学研究所的一部分。参与者是从伦敦皇家自由医院和伦敦国家神经病学与神经外科国家医院建立的数据库中招募的。对24例中度PD患者进行了资格评估,其中23例进入了研究。其中,有18名患者完成了研究。排除1例患者(腰椎穿刺失败),退出4例患者(主要是腰椎穿刺相关并发症)。所有数据分析均于2018年11月1日至12月14日进行。主要结果和措施186天的主要结果是脑脊液中氨溴索的检出和脑脊液GCase活性的变化。结果完成研究的18名参与者(15名男性[83.3%];平均[SD]年龄,60.2 [9.7]岁)中,有17名参与者(其中8名具有GBA1突变,9名没有GBA1突变)被包括在主要分析中。在第0天和第186天之间,观察到CSF中氨溴索水平增加156 ng / mL(较低的95%置信限,129 ng / mL; P <.001)。CSF GCase活性降低了19%(每小时0.059 nmol / mL; 95%CI,-0.115至-0.002; P = .04)。氨溴索疗法耐受良好,无严重不良事件。CSFα-突触核蛋白浓度增加50 pg / mL(13%)(95%CI,14-87; P = 0.01),CSF GCase蛋白水平增加88 ng / mol(35%)观察到(95%CI,40-137; P = .002)。运动障碍学会帕金森病统一评分量表第3部分的平均(SD)得分降低了6.8(7)。1)分(95%CI,-10.4至-3.1; P = 0.001)。在有和没有GBA1突变的患者中观察到了这些变化。结论与相关性研究结果表明氨溴索治疗是安全且耐受性良好的。实现了脑脊液渗透和氨溴索的靶定参与,并且脑脊液α-突触核蛋白水平增加。需要安慰剂对照的临床试验来检查氨溴索治疗是否与PD自然进程的改变有关。试验注册ClinicalTrials.gov标识符:NCT02941822;EudraCT标识符:2015-002571-24。和脑脊液α-突触核蛋白水平升高。需要安慰剂对照的临床试验来检查氨溴索治疗是否与PD自然进程的改变有关。试验注册ClinicalTrials.gov标识符:NCT02941822;EudraCT标识符:2015-002571-24。和脑脊液α-突触核蛋白水平升高。需要安慰剂对照的临床试验来检查氨溴索治疗是否与PD自然进程的改变有关。试验注册ClinicalTrials.gov标识符:NCT02941822;EudraCT标识符:2015-002571-24。











































 京公网安备 11010802027423号
京公网安备 11010802027423号