当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics and Kinetics of Gas-Phase CO Oxidation on the Scandium Monoxide Carbonyl Complexes.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jpca.9b10659 Zhiling Liu 1 , Lina Hou 1 , Ya Li 1 , Gang Li 2 , Zhengbo Qin 3 , Hai-Shun Wu 1 , Jianfeng Jia 1 , Hua Xie 2 , Zichao Tang 4
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jpca.9b10659 Zhiling Liu 1 , Lina Hou 1 , Ya Li 1 , Gang Li 2 , Zhengbo Qin 3 , Hai-Shun Wu 1 , Jianfeng Jia 1 , Hua Xie 2 , Zichao Tang 4
Affiliation
|
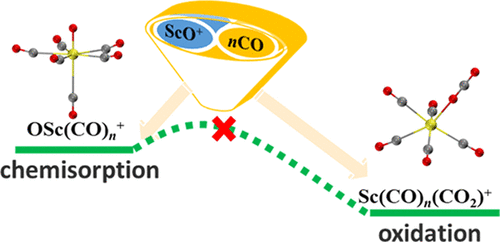
The CO chemisorption onto the ScO+ cation was investigated using infrared photodissociation spectroscopy combined with density functional theory calculations. The spectra were recorded in the CO stretching vibrational region for the OSc(CO)n+ (n = 4-6) complex series. Comparisons of the experimental spectra with the simulated ones have established the geometries and present strong evidence that all of the CO ligands are chemisorbed, which could not be readily oxidized by scandium monoxide core into CO2. Complementary calculations demonstrate that, regardless of the thermodynamic feasibility, the CO oxidation on the scandium monoxide carbonyl complexes is kinetically unfavorable due to the significant barriers involved in the CO oxidation process. Nevertheless, the consecutive CO adsorption has a positive influence on the Sc-O bond activation.
中文翻译:

一氧化dium羰基配合物气相CO氧化的热力学和动力学
使用红外光解离光谱结合密度泛函理论计算研究了CO在ScO +阳离子上的化学吸附。光谱记录在OSc(CO)n +(n = 4-6)配合物系列的CO拉伸振动区域中。实验光谱与模拟光谱的比较已经确定了几何形状,并提供了有力的证据表明所有的CO配体都是化学吸附的,一氧化mon核不易将其氧化成CO2。补充计算表明,不管热力学可行性如何,一氧化oxidation羰基配合物上的CO氧化在动力学上都是不利的,因为在CO氧化过程中存在重大障碍。尽管如此,连续的CO吸附对Sc-O键的活化有积极影响。
更新日期:2020-01-23
中文翻译:

一氧化dium羰基配合物气相CO氧化的热力学和动力学
使用红外光解离光谱结合密度泛函理论计算研究了CO在ScO +阳离子上的化学吸附。光谱记录在OSc(CO)n +(n = 4-6)配合物系列的CO拉伸振动区域中。实验光谱与模拟光谱的比较已经确定了几何形状,并提供了有力的证据表明所有的CO配体都是化学吸附的,一氧化mon核不易将其氧化成CO2。补充计算表明,不管热力学可行性如何,一氧化oxidation羰基配合物上的CO氧化在动力学上都是不利的,因为在CO氧化过程中存在重大障碍。尽管如此,连续的CO吸附对Sc-O键的活化有积极影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号