当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-Ugi Transformations for the Access to Pyrrolobenzodiazepine Scaffolds with Different Degrees of Unsaturation.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.joc.9b02995 Pablo Pertejo 1 , Israel Carreira-Barral 1 , Pablo Peña-Calleja 1 , Roberto Quesada 1 , María García-Valverde 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.joc.9b02995 Pablo Pertejo 1 , Israel Carreira-Barral 1 , Pablo Peña-Calleja 1 , Roberto Quesada 1 , María García-Valverde 1
Affiliation
|
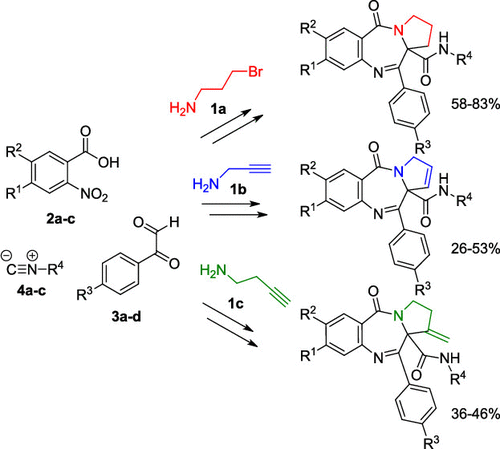
The synthesis of three novel families of pyrrolo[2,1-c][1,4]benzodiazepine-5-ones is described. The compounds were prepared according to a three-step sequence, involving an Ugi reaction, building of the pyrrolo nucleus, and reduction-cyclization to the corresponding diazepine. Depending on the amine employed in the synthesis of the Ugi adducts, different unsaturation degrees could be obtained in the pyrrolo ring (saturated or with endo or exo unsaturations), a key feature determining their biological activity, as it affects the affinity of the pyrrolobenzodiazepines toward DNA and thus their cytotoxicity. This synthetic methodology represents a significant improvement with respect to those described in the literature so far, as it uses inexpensive and commercially available starting materials without needing derivatization or the use of protecting groups.
中文翻译:

Ugi改造后获得了不同程度的不饱和度的吡咯并苯并二氮杂pine骨架。
描述了三个新颖的吡咯并[2,1-c] [1,4]苯并二氮杂-5-酮家族的合成。按照三步法制备化合物,包括Ugi反应,吡咯并核的构建以及还原环化成相应的二氮杂pine。取决于用于合成Ugi加合物的胺,吡咯并环(饱和的或具有内或外或不饱和的)可以得到不同的不饱和度,这是决定其生物学活性的关键特征,因为它影响吡咯并苯并二氮杂卓对DNA及其细胞毒性。相对于迄今为止的文献中描述的方法,这种综合方法论已取得了重大进步,
更新日期:2020-01-23
中文翻译:

Ugi改造后获得了不同程度的不饱和度的吡咯并苯并二氮杂pine骨架。
描述了三个新颖的吡咯并[2,1-c] [1,4]苯并二氮杂-5-酮家族的合成。按照三步法制备化合物,包括Ugi反应,吡咯并核的构建以及还原环化成相应的二氮杂pine。取决于用于合成Ugi加合物的胺,吡咯并环(饱和的或具有内或外或不饱和的)可以得到不同的不饱和度,这是决定其生物学活性的关键特征,因为它影响吡咯并苯并二氮杂卓对DNA及其细胞毒性。相对于迄今为止的文献中描述的方法,这种综合方法论已取得了重大进步,











































 京公网安备 11010802027423号
京公网安备 11010802027423号