当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fast Li+ Conduction Mechanism and Interfacial Chemistry of a NASICON/Polymer Composite Electrolyte
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-13 , DOI: 10.1021/jacs.9b12233 Nan Wu 1, 2 , Po-Hsiu Chien 3, 4 , Yutao Li 1 , Andrei Dolocan 1 , Henghui Xu 1 , Biyi Xu 1 , Nicholas S Grundish 1 , Haibo Jin 2 , Yan-Yan Hu 3, 4 , John B Goodenough 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-13 , DOI: 10.1021/jacs.9b12233 Nan Wu 1, 2 , Po-Hsiu Chien 3, 4 , Yutao Li 1 , Andrei Dolocan 1 , Henghui Xu 1 , Biyi Xu 1 , Nicholas S Grundish 1 , Haibo Jin 2 , Yan-Yan Hu 3, 4 , John B Goodenough 1
Affiliation
|
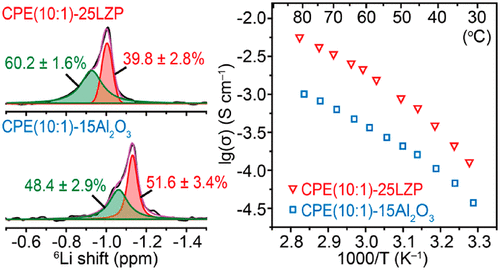
The unclear Li+ local environment and Li+ conduction mechanism in solid polymer electrolytes, especially in a ceramic/polymer composite electrolyte, hinder the design and development of a new composite electrolyte. Moreover, both the low room-temperature Li+ conductivity and large interfacial resistance with a metallic lithium anode of a polymer membrane limit its application below a relatively high temperature. Here we have identified the Li+ distribution and Li+ transport mechanism in a composite polymer electrolyte by investigating a new solid poly(ethylene oxide) (PEO)-based NASICON-LiZr2(PO4)3 composite with 7Li relaxation time and 6Li→7Li trace-exchange NMR measurements. The Li+ population of the two local environments in the composite electrolytes depends on the Li-salt concentration and the amount of ceramic filler. A composite electrolyte with a [EO]/[Li+] ratio n = 10 and 25 wt% LZP filler has a high Li+ conductivity of 1.2 × 10-4 S cm-1 at 30 oC and a low activation energy owing to the additional Li+ in the mobile A2 environment. Moreover, an in-situ-formed solid electrolyte interphase layer from the reaction between LiZr2(PO4)3 and a metallic lithium anode stabilized the Li/composite-electrolyte interface and reduced the interfacial resistance, which provided a symmetric Li/Li cell, and all-solid-state Li/LiFePO4 and Li/LiNi0.8Co0.1Mn0.1O2 cells a good cycling performance at 40 oC.
中文翻译:

NASICON/聚合物复合电解质的快速锂离子传导机制和界面化学
固态聚合物电解质,尤其是陶瓷/聚合物复合电解质中,Li+局部环境和Li+传导机制不清楚,阻碍了新型复合电解质的设计和开发。此外,低室温 Li+ 电导率和与聚合物膜的金属锂阳极的大界面电阻都限制了其在相对较高温度以下的应用。在这里,我们通过研究具有 7Li 弛豫时间和 6Li→7Li 痕量交换的新型固体聚环氧乙烷 (PEO) 基 NASICON-LiZr2(PO4)3 复合材料,确定了复合聚合物电解质中的 Li+ 分布和 Li+ 传输机制核磁共振测量。复合电解质中两种局部环境的 Li+ 数量取决于锂盐浓度和陶瓷填料的量。[EO]/[Li+] 比率 n = 10 和 25 wt% LZP 填料的复合电解质在 30 oC 下具有 1.2 × 10-4 S cm-1 的高 Li+ 电导率和低活化能,这是由于额外的 Li+在移动 A2 环境中。此外,由 LiZr2(PO4)3 与金属锂负极反应形成的固体电解质中间相层稳定了 Li/复合电解质界面并降低了界面电阻,从而提供了对称的 Li/Li 电池,并且全固态 Li/LiFePO4 和 Li/LiNi0.8Co0.1Mn0.1O2 电池在 40 oC 下具有良好的循环性能。
更新日期:2020-01-13
中文翻译:

NASICON/聚合物复合电解质的快速锂离子传导机制和界面化学
固态聚合物电解质,尤其是陶瓷/聚合物复合电解质中,Li+局部环境和Li+传导机制不清楚,阻碍了新型复合电解质的设计和开发。此外,低室温 Li+ 电导率和与聚合物膜的金属锂阳极的大界面电阻都限制了其在相对较高温度以下的应用。在这里,我们通过研究具有 7Li 弛豫时间和 6Li→7Li 痕量交换的新型固体聚环氧乙烷 (PEO) 基 NASICON-LiZr2(PO4)3 复合材料,确定了复合聚合物电解质中的 Li+ 分布和 Li+ 传输机制核磁共振测量。复合电解质中两种局部环境的 Li+ 数量取决于锂盐浓度和陶瓷填料的量。[EO]/[Li+] 比率 n = 10 和 25 wt% LZP 填料的复合电解质在 30 oC 下具有 1.2 × 10-4 S cm-1 的高 Li+ 电导率和低活化能,这是由于额外的 Li+在移动 A2 环境中。此外,由 LiZr2(PO4)3 与金属锂负极反应形成的固体电解质中间相层稳定了 Li/复合电解质界面并降低了界面电阻,从而提供了对称的 Li/Li 电池,并且全固态 Li/LiFePO4 和 Li/LiNi0.8Co0.1Mn0.1O2 电池在 40 oC 下具有良好的循环性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号