当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vitro and In Vivo Study of Amphotericin B Formulation with Quaternized Bioreducible Lipidoids
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-01-22 , DOI: 10.1021/acsbiomaterials.9b01722 Fang Liu 1, 2 , Liu Yang 1 , Yamin Li 1 , Ashlee Junier 3 , Feihe Ma 1 , Jinjin Chen 1 , Haobo Han 1, 4 , Zachary Glass 1 , Xuewei Zhao 1 , Carol A Kumamoto 3 , Hong Sang 2 , Qiaobing Xu 1
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-01-22 , DOI: 10.1021/acsbiomaterials.9b01722 Fang Liu 1, 2 , Liu Yang 1 , Yamin Li 1 , Ashlee Junier 3 , Feihe Ma 1 , Jinjin Chen 1 , Haobo Han 1, 4 , Zachary Glass 1 , Xuewei Zhao 1 , Carol A Kumamoto 3 , Hong Sang 2 , Qiaobing Xu 1
Affiliation
|
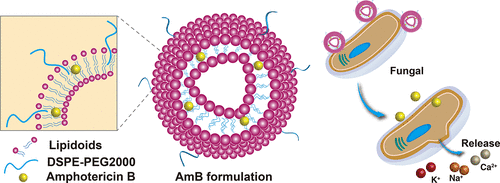
Invasive fungal infections are well-known causes of morbidity and mortality in immunocompromised patients. Amphotericin B (AmB) is a polyene fungicidal agent with excellent properties of the broad antifungal spectrum, high activity, and relatively rare drug resistance. However, significant toxicities limit the clinical application of AmB and its conventional formulation AmB deoxycholate (Fungizone). Here we investigated nanoparticle formulations of AmB using synthetic biodegradable lipidoids and evaluated their stability, in vitro antifungal efficacy, and in vivo toxicity and pharmacokinetics. We found that the AmB formulated using a mixture of quaternized lipidoid (Q78-O14B) and DSPE-PEG2000 has the size around 70–100 nm and is stable during storage. The formulation showed no hemotoxicity to red blood cells (RBCs) in vitro. It also possesses the highest antifungal activity (in vitro) and lowest toxicity (both in vitro and in vivo). These metrics are significantly superior to the commercial antifungal product Fungizone. Meanwhile, AmB/Q78-O14B-P exhibited prolonged blood circulation in comparison to Fungizone in vivo. In AmB/Q78-O14B-P formulation, AmB was still detectable in the liver, spleen, and lung tissues with a concentration above the minimum inhibitory concentrations 72 h after low-dose intravenous injection. Based on these results, AmB in lipidoid nanoparticle formulation may produce sustained antifungal activity against blood-borne and systemic organ infections. Moreover, the new AmB formulation showed low nephrotoxicity and hepatotoxicity in rats even at high doses, allowing a dramatically wider and safer therapeutic window than Fungizone. This method provides a means to develop much needed antifungal agents that will be more therapeutically efficacious, more affordable (than AmBisome), and less toxic (than Fungizone) for the treatment of systemic fungal infections.
中文翻译:

具有季铵化生物还原性脂质的两性霉素 B 制剂的体外和体内研究
侵袭性真菌感染是免疫功能低下患者发病和死亡的众所周知的原因。两性霉素B(AmB)是一种多烯类杀菌剂,具有抗真菌谱广、活性高、耐药性相对较少等优良特性。然而,显着的毒性限制了 AmB 及其常规制剂 AmB 脱氧胆酸盐(Fungizone)的临床应用。在这里,我们使用合成的可生物降解类脂质研究了 AmB 的纳米颗粒制剂,并评估了它们的稳定性、体外抗真菌功效和体内毒性和药代动力学。我们发现使用季铵化类脂质 (Q78-O14B) 和 DSPE-PEG2000 的混合物配制的 AmB 的尺寸约为 70-100 nm,并且在储存期间是稳定的。该制剂在体外对红细胞 (RBC) 没有血液毒性。它还具有最高的抗真菌活性(体外)和最低的毒性(体外和体内)。这些指标明显优于商业抗真菌产品 Fungizone。同时,与体内的 Fungizone 相比,AmB/Q78-O14B-P 表现出延长的血液循环。在 AmB/Q78-O14B-P 制剂中,在低剂量静脉注射后 72 小时,仍可在肝、脾和肺组织中检测到 AmB,其浓度高于最低抑制浓度。基于这些结果,类脂质纳米颗粒制剂中的 AmB 可能对血液传播和全身器官感染产生持续的抗真菌活性。此外,即使在高剂量下,新的 AmB 制剂对大鼠的肾毒性和肝毒性也较低,与 Fungizone 相比,其治疗窗口显着更宽、更安全。这种方法提供了一种开发急需的抗真菌剂的方法,这些抗真菌剂在治疗全身性真菌感染时将更有效、更实惠(比 AmBisome)、毒性更小(比 Fungizone)。
更新日期:2020-01-23
中文翻译:

具有季铵化生物还原性脂质的两性霉素 B 制剂的体外和体内研究
侵袭性真菌感染是免疫功能低下患者发病和死亡的众所周知的原因。两性霉素B(AmB)是一种多烯类杀菌剂,具有抗真菌谱广、活性高、耐药性相对较少等优良特性。然而,显着的毒性限制了 AmB 及其常规制剂 AmB 脱氧胆酸盐(Fungizone)的临床应用。在这里,我们使用合成的可生物降解类脂质研究了 AmB 的纳米颗粒制剂,并评估了它们的稳定性、体外抗真菌功效和体内毒性和药代动力学。我们发现使用季铵化类脂质 (Q78-O14B) 和 DSPE-PEG2000 的混合物配制的 AmB 的尺寸约为 70-100 nm,并且在储存期间是稳定的。该制剂在体外对红细胞 (RBC) 没有血液毒性。它还具有最高的抗真菌活性(体外)和最低的毒性(体外和体内)。这些指标明显优于商业抗真菌产品 Fungizone。同时,与体内的 Fungizone 相比,AmB/Q78-O14B-P 表现出延长的血液循环。在 AmB/Q78-O14B-P 制剂中,在低剂量静脉注射后 72 小时,仍可在肝、脾和肺组织中检测到 AmB,其浓度高于最低抑制浓度。基于这些结果,类脂质纳米颗粒制剂中的 AmB 可能对血液传播和全身器官感染产生持续的抗真菌活性。此外,即使在高剂量下,新的 AmB 制剂对大鼠的肾毒性和肝毒性也较低,与 Fungizone 相比,其治疗窗口显着更宽、更安全。这种方法提供了一种开发急需的抗真菌剂的方法,这些抗真菌剂在治疗全身性真菌感染时将更有效、更实惠(比 AmBisome)、毒性更小(比 Fungizone)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号