当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem Access to Acridones and their Fused Derivatives: [1+2+3] Annulation of Isocyanides with Unsaturated Carbonyls
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-10 , DOI: 10.1002/adsc.201901560 Ling‐Juan Zhang 1 , Wenhui Yang 1 , Zhongyan Hu 2 , Xian‐Ming Zhang 1 , Xianxiu Xu 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-10 , DOI: 10.1002/adsc.201901560 Ling‐Juan Zhang 1 , Wenhui Yang 1 , Zhongyan Hu 2 , Xian‐Ming Zhang 1 , Xianxiu Xu 2
Affiliation
|
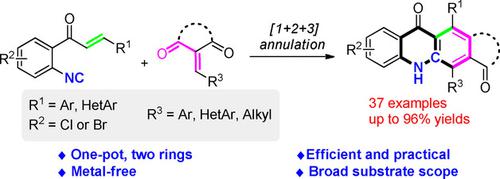
A wide range of acridones and their cyclo[b ]‐fused derivatives are efficiently constructed by a double annulation of o ‐enoyl arylisocyanides with α , β ‐unsaturated carbonyls under simple metal‐free condition. This protocol is general, efficient and practical, featuring the successive formation of two rings by a one‐pot domino transformation. A tandem process involing an isocyanide‐based [1+4] cycloaddition, an aminofuran‐based intramolecular [4+2] cycloaddition, ring opening and aromatization is proposed for the transformation.
中文翻译:

串联获取Ac啶酮及其熔融衍生物:[1 + 2 + 3]异氰酸酯与不饱和羰基的环化
在简单的无金属条件下,通过邻烯基芳基异氰酸酯与α,β-不饱和羰基的双环化,可以有效地构建各种各样的a烯及其环[ b ]稠合衍生物。该协议是通用,高效和实用的,其特点是通过一锅多米诺骨牌变换连续形成两个环。提出了将异氰酸酯基[1 + 4]环加成,氨基呋喃基分子内[4 + 2]环加成,开环和芳构化等串联过程。
更新日期:2020-01-10
中文翻译:

串联获取Ac啶酮及其熔融衍生物:[1 + 2 + 3]异氰酸酯与不饱和羰基的环化
在简单的无金属条件下,通过邻烯基芳基异氰酸酯与α,β-不饱和羰基的双环化,可以有效地构建各种各样的a烯及其环[ b ]稠合衍生物。该协议是通用,高效和实用的,其特点是通过一锅多米诺骨牌变换连续形成两个环。提出了将异氰酸酯基[1 + 4]环加成,氨基呋喃基分子内[4 + 2]环加成,开环和芳构化等串联过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号