当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Chiral 5‐Aryl‐2‐oxazolidinones via Halohydrin Dehalogenase‐Catalyzed Enantio‐ and Regioselective Ring‐Opening of Styrene Oxides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-28 , DOI: 10.1002/adsc.201901412 Nanwei Wan 1, 2 , Xiaoying Zhou 1, 2 , Ran Ma 1, 2 , Jiawei Tian 1, 2 , Huihui Wang 1, 2 , Baodong Cui 1, 2 , Wenyong Han 1, 2 , Yongzheng Chen 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-28 , DOI: 10.1002/adsc.201901412 Nanwei Wan 1, 2 , Xiaoying Zhou 1, 2 , Ran Ma 1, 2 , Jiawei Tian 1, 2 , Huihui Wang 1, 2 , Baodong Cui 1, 2 , Wenyong Han 1, 2 , Yongzheng Chen 1, 2
Affiliation
|
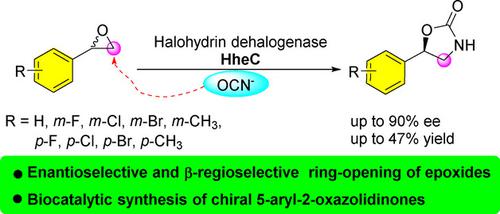
An efficient biocatalytic approach for enantio‐ and regioselective ring‐opening of styrene oxides with cyanate was developed by using the halohydrin dehalogenase HheC from Agrobacterium radiobacter AD1, generating the corresponding chiral 5‐aryl‐2‐oxazolidinones in up to 47% yield and 90% ee. Additionally, the origin of enantioselectivity and regioselectivity of the HheC‐catalyzed cyanate‐mediated ring‐opening process was uncovered by single enantiomer bioconversions and molecular docking study.
中文翻译:

卤代醇脱卤酶催化的苯乙烯氧化物对映和区域选择性开环合成手性5-芳基-2-恶唑烷酮
利用土壤杆菌放射杆菌AD1的卤代醇脱卤酶HheC,开发了一种有效的生物催化方法,用于对苯乙烯氧化物与氰酸酯的对映和区域选择性开环,生成相应的手性5-芳基-2-恶唑烷酮,产率高达47%,产率为90% ee。此外,单个对映异构体的生物转化和分子对接研究未发现HheC催化的氰酸酯介导的开环过程的对映选择性和区域选择性的起源。
更新日期:2020-01-29
中文翻译:

卤代醇脱卤酶催化的苯乙烯氧化物对映和区域选择性开环合成手性5-芳基-2-恶唑烷酮
利用土壤杆菌放射杆菌AD1的卤代醇脱卤酶HheC,开发了一种有效的生物催化方法,用于对苯乙烯氧化物与氰酸酯的对映和区域选择性开环,生成相应的手性5-芳基-2-恶唑烷酮,产率高达47%,产率为90% ee。此外,单个对映异构体的生物转化和分子对接研究未发现HheC催化的氰酸酯介导的开环过程的对映选择性和区域选择性的起源。









































 京公网安备 11010802027423号
京公网安备 11010802027423号