当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halogen-bonding-induced diverse aggregation of 4,5-diiodo-1,2,3-triazolium salts with different anions.
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-01-13 , DOI: 10.3762/bjoc.16.10 Xingyu Xu 1 , Shiqing Huang 1 , Zengyu Zhang 1 , Lei Cao 1 , Xiaoyu Yan 1
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-01-13 , DOI: 10.3762/bjoc.16.10 Xingyu Xu 1 , Shiqing Huang 1 , Zengyu Zhang 1 , Lei Cao 1 , Xiaoyu Yan 1
Affiliation
|
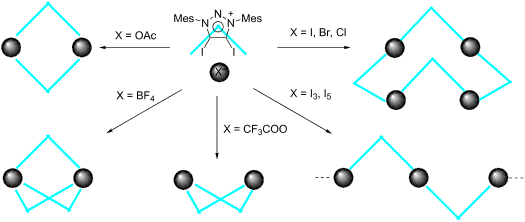
The synthesis of 4,5-diiodo-1,3-dimesityl-1,2,3-triazolium salts with different anions have been developed. These triazolium salts show diverse aggregation via halogen bonding between C-I bonds and anions. Triazolium with halide anions exists as a tetramer with saddle conformation. Triazolium tetrafluoroborate exists as a trimer with Chinese lantern shape conformation. Triazolium trifluoroacetate and acetate exist as dimers, respectively, while the former shows boat conformation and the latter forms rectangle conformation. Triazolium salts form a linear polymer with polyiodide.
更新日期:2020-01-13











































 京公网安备 11010802027423号
京公网安备 11010802027423号