Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic analysis of sequential metabolism of triazolam and its extrapolation to humans using an entero-hepatic two-organ microphysiological system.
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9lc00884e Hiroshi Arakawa 1 , Shinji Sugiura 2 , Takumi Kawanishi 1 , Kazumi Shin 2 , Hiroko Toyoda 3 , Taku Satoh 3 , Yasuyuki Sakai 4 , Toshiyuki Kanamori 2 , Yukio Kato 1
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9lc00884e Hiroshi Arakawa 1 , Shinji Sugiura 2 , Takumi Kawanishi 1 , Kazumi Shin 2 , Hiroko Toyoda 3 , Taku Satoh 3 , Yasuyuki Sakai 4 , Toshiyuki Kanamori 2 , Yukio Kato 1
Affiliation
|
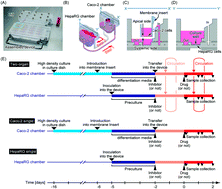
The microphysiological system (MPS) is a promising tool for predicting drug disposition in humans, although limited information is available on the quantitative assessment of sequential drug metabolism in MPS and its extrapolation to humans. In the present study, we first constructed a mechanism-based pharmacokinetic model for triazolam (TRZ) and its metabolites in the entero-hepatic two-organ MPS, composed of intestinal Caco-2 and hepatic HepaRG cells, and attempted to extrapolate the kinetic information obtained with the MPS to the plasma concentration profiles in humans. In the two-organ MPS and HepaRG single culture systems, TRZ was found to be metabolized into α- and 4-hydroxytriazolam and their respective glucuronides. All these metabolites were almost completely reduced in the presence of a CYP3A inhibitor, itraconazole, confirming sequential phase I and II metabolism. Both pharmacokinetic model-dependent and -independent analyses were performed, providing consistent results regarding the metabolic activity of TRZ: clearance of glucuronidation metabolites in the two-organ MPS was higher than that in the single culture system. The plasma concentration profile of TRZ and its two hydroxy metabolites in humans was quantitatively simulated based on the pharmacokinetic model, by incorporating several scaling factors representing quantitative gaps between the MPS and humans. Thus, the present study provided the first quantitative extrapolation of sequential drug metabolism in humans by combining MPS and pharmacokinetic modeling.
中文翻译:

使用肠肝两器官微生理系统对三唑仑连续代谢及其外推到人体的动力学进行分析。
微生理系统(MPS)是预测人类药物分布的有前途的工具,尽管有关MPS中药物顺序代谢及其外推给人类的定量评估信息有限。在本研究中,我们首先构建了基于机理的三唑仑(TRZ)及其代谢物在肠肝两器官MPS中的药代动力学模型,该肠肝两器官MPS由肠Caco-2和肝HepaRG细胞组成,并试图推断动力学信息用MPS获得的血浆浓度曲线。在两器官MPS和HepaRG单一培养系统中,发现TRZ被代谢为α-和4-羟基三唑仑及其各自的葡萄糖醛酸苷。在CYP3A抑制剂伊曲康唑存在下,所有这些代谢物几乎都被完全还原,确认相继的I和II期代谢。进行了药代动力学模型依赖性分析和非依赖性模型分析,就TRZ的代谢活性提供了一致的结果:双器官MPS中葡萄糖醛酸化代谢产物的清除率高于单一培养系统。基于药代动力学模型,通过纳入代表MPS和人类之间定量差距的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢的首次定量外推提供了条件。就TRZ的代谢活性提供了一致的结果:在两个器官的MPS中,葡萄糖醛酸化代谢产物的清除率高于在单个培养系统中。基于药代动力学模型,通过纳入代表MPS和人类之间定量差距的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢的首次定量外推提供了条件。就TRZ的代谢活性提供了一致的结果:在两个器官的MPS中,葡萄糖醛酸化代谢产物的清除率高于在单个培养系统中。基于药代动力学模型,通过纳入代表MPS和人类之间定量缺口的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢的首次定量外推提供了条件。基于药代动力学模型,通过纳入代表MPS和人类之间定量差距的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢提供了第一个定量推断。基于药代动力学模型,通过纳入代表MPS和人类之间定量差距的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢的首次定量外推提供了条件。
更新日期:2020-02-13
中文翻译:

使用肠肝两器官微生理系统对三唑仑连续代谢及其外推到人体的动力学进行分析。
微生理系统(MPS)是预测人类药物分布的有前途的工具,尽管有关MPS中药物顺序代谢及其外推给人类的定量评估信息有限。在本研究中,我们首先构建了基于机理的三唑仑(TRZ)及其代谢物在肠肝两器官MPS中的药代动力学模型,该肠肝两器官MPS由肠Caco-2和肝HepaRG细胞组成,并试图推断动力学信息用MPS获得的血浆浓度曲线。在两器官MPS和HepaRG单一培养系统中,发现TRZ被代谢为α-和4-羟基三唑仑及其各自的葡萄糖醛酸苷。在CYP3A抑制剂伊曲康唑存在下,所有这些代谢物几乎都被完全还原,确认相继的I和II期代谢。进行了药代动力学模型依赖性分析和非依赖性模型分析,就TRZ的代谢活性提供了一致的结果:双器官MPS中葡萄糖醛酸化代谢产物的清除率高于单一培养系统。基于药代动力学模型,通过纳入代表MPS和人类之间定量差距的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢的首次定量外推提供了条件。就TRZ的代谢活性提供了一致的结果:在两个器官的MPS中,葡萄糖醛酸化代谢产物的清除率高于在单个培养系统中。基于药代动力学模型,通过纳入代表MPS和人类之间定量差距的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢的首次定量外推提供了条件。就TRZ的代谢活性提供了一致的结果:在两个器官的MPS中,葡萄糖醛酸化代谢产物的清除率高于在单个培养系统中。基于药代动力学模型,通过纳入代表MPS和人类之间定量缺口的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢的首次定量外推提供了条件。基于药代动力学模型,通过纳入代表MPS和人类之间定量差距的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢提供了第一个定量推断。基于药代动力学模型,通过纳入代表MPS和人类之间定量差距的几个比例因子,对TRZ及其两种羟基代谢物的血浆浓度分布进行了定量模拟。因此,本研究通过结合MPS和药代动力学模型,为人类连续药物代谢的首次定量外推提供了条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号