当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cryo-EM Structure of Full-length HIV-1 Env Bound With the Fab of Antibody PG16.
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jmb.2019.11.028 Junhua Pan 1 , Hanqin Peng 1 , Bing Chen 1 , Stephen C Harrison 2
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jmb.2019.11.028 Junhua Pan 1 , Hanqin Peng 1 , Bing Chen 1 , Stephen C Harrison 2
Affiliation
|
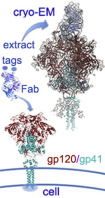
The HIV-1 envelope protein (Env) is the target of neutralizing antibodies and the template for vaccine immunogen design. The dynamic conformational equilibrium of trimeric Env influences its antigenicity and potential immunogenicity. Antibodies that bind at the trimer apex stabilize a "closed" conformation characteristic of the most difficult to neutralize isolates. A goal of vaccine development is therefore to mimic the closed conformation in a designed immunogen. A disulfide-stabilized, trimeric Env ectodomain-the "SOSIP" construct-has many of the relevant properties; it is also particularly suitable for structure determination. Some single-molecule studies have, however, suggested that the SOSIP trimer is not a good representation of Env on the surface of a virion or an infected cell. We isolated Env (fully cleaved to gp120 and gp41) from the surface of expressing cells using tagged, apex-binding Fab PG16 and determined the structure of the PG16-Env complex by cryo-EM to an overall resolution of 4.6 Å. Placing the only purification tag on the Fab ensured that the isolated Env was continuously stabilized in its closed, native conformation. The Env structure in this complex corresponds closely to the SOSIP structures determined by both x-ray crystallography and cryo-EM. Although the membrane-interacting elements are not resolved in our reconstruction, we can make inferences about the connection between ectodomain and membrane-proximal external region (MPER) by reference to the published cryo-tomography structure of an Env "spike" and the NMR structure of the MPER-transmembrane segment. We discuss these results in view of the conflicting interpretations in the literature.
中文翻译:

全长HIV-1 Env的低温EM结构与抗体PG16的Fab结合。
HIV-1包膜蛋白(Env)是中和抗体的靶标,也是疫苗免疫原设计的模板。三聚体Env的动态构象平衡影响其抗原性和潜在的免疫原性。在三聚体顶端结合的抗体可稳定最难中和的分离物的“封闭”构象特征。因此,疫苗开发的目标是模仿设计的免疫原中的封闭构象。二硫键稳定的三聚Env胞外域-“ SOSIP”构建体-具有许多相关特性;它也特别适用于结构确定。但是,一些单分子研究表明,SOSIP三聚体不能很好地代表病毒颗粒或感染细胞表面上的Env。我们使用标记的,顶点结合的Fab PG16从表达细胞的表面分离了Env(完全裂解为gp120和gp41),并通过冷冻-EM测定了PG16-Env复合物的结构,总分辨率为4.6。将唯一的纯化标签放置在Fab上可确保分离的Env持续稳定在其封闭的天然构象中。该复合物中的Env结构与通过X射线晶体学和cryo-EM确定的SOSIP结构紧密对应。尽管在我们的重建中无法解决膜相互作用元素,但我们可以参考已发表的Env“峰”的冷冻断层扫描结构和NMR结构来推断胞外域与膜近端外部区域(MPER)之间的连接MPER跨膜片段的片段。
更新日期:2020-01-13
中文翻译:

全长HIV-1 Env的低温EM结构与抗体PG16的Fab结合。
HIV-1包膜蛋白(Env)是中和抗体的靶标,也是疫苗免疫原设计的模板。三聚体Env的动态构象平衡影响其抗原性和潜在的免疫原性。在三聚体顶端结合的抗体可稳定最难中和的分离物的“封闭”构象特征。因此,疫苗开发的目标是模仿设计的免疫原中的封闭构象。二硫键稳定的三聚Env胞外域-“ SOSIP”构建体-具有许多相关特性;它也特别适用于结构确定。但是,一些单分子研究表明,SOSIP三聚体不能很好地代表病毒颗粒或感染细胞表面上的Env。我们使用标记的,顶点结合的Fab PG16从表达细胞的表面分离了Env(完全裂解为gp120和gp41),并通过冷冻-EM测定了PG16-Env复合物的结构,总分辨率为4.6。将唯一的纯化标签放置在Fab上可确保分离的Env持续稳定在其封闭的天然构象中。该复合物中的Env结构与通过X射线晶体学和cryo-EM确定的SOSIP结构紧密对应。尽管在我们的重建中无法解决膜相互作用元素,但我们可以参考已发表的Env“峰”的冷冻断层扫描结构和NMR结构来推断胞外域与膜近端外部区域(MPER)之间的连接MPER跨膜片段的片段。



























 京公网安备 11010802027423号
京公网安备 11010802027423号