当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiol antioxidants sensitize malabaricone C induced cancer cell death via reprogramming redox sensitive p53 and NF-κB proteins in vitro and in vivo.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.freeradbiomed.2020.01.011 Mrityunjay Tyagi 1 , Ajay Kumar Bauri 2 , Subrata Chattopadhyay 2 , Birija Sankar Patro 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.freeradbiomed.2020.01.011 Mrityunjay Tyagi 1 , Ajay Kumar Bauri 2 , Subrata Chattopadhyay 2 , Birija Sankar Patro 1
Affiliation
|
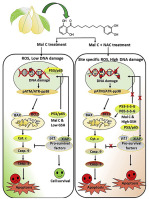
Specific focus on "redox cancer therapy" by targeting drugs to redox homeostasis of the cancer cells is growing rapidly. Recent clinical studies showed that N-acetyl cysteine (NAC) treatment significantly decreased the metabolic heterogeneity and reduced Ki67 (a proliferation marker) with simultaneous enhancement in apoptosis of tumor cells in patients. However, it is not yet precisely known how thiol antioxidants enhance killing of cancer cells in a context dependent manner. To this end, we showed that a dietary compound, malabaricone C (mal C) generated copious amounts of reactive oxygen species (ROS) and also reduced GSH level in lung cancer cells. Paradoxically, although antioxidants supplementation reduced mal C-induced ROS, thiol-antioxidants (NAC/GSH) restored intracellular GSH level but enhanced DNA DSBs and apoptotic cell death induced by mal C. Our results unraveled two tightly coupled biochemical mechanisms attributing this sensitization process by thiol antioxidants. Firstly, thiol antioxidants enable the "catechol-quinone redox cycle" of mal C and ameliorate ROS generation and bio-molecular damage (DNA and protein). Secondly, thiol antioxidants cause rapid glutathionylation of transcription factors [p53, p65 (NF-κB) etc.], oxidized by mal C, and abrogates their nuclear sequestration and transcription of the anti-apoptotic genes. Furthermore, analyses of the mitochondrial fractions of p53 expressing and silenced cells revealed that cytoplasmic accumulation of glutathionylated p53 (p53-SSG) triggers a robust mitochondrial death process. Interestingly, mutation of redox sensitive cysteine residues at 124, 141 and 182 position in p53 significantly reduces mal C plus NAC mediated sensitization of cancer cells. The preclinical results, in two different tumor models in mice, provides further support our conclusion that NAC is able to sensitize mal C induced suppression of tumor growth in vivo.
中文翻译:

硫醇抗氧化剂通过在体外和体内对氧化还原敏感的p53和NF-κB蛋白进行重新编程,使马拉巴利康C诱导的癌细胞死亡敏感。
通过将药物靶向癌细胞的氧化还原稳态,对“氧化还原癌症疗法”的特别关注正在迅速增长。最近的临床研究表明,N-乙酰半胱氨酸(NAC)治疗可显着降低代谢异质性并降低Ki67(一种增殖标志物),并同时增强患者肿瘤细胞的凋亡。然而,尚不确切知道硫醇抗氧化剂如何以上下文相关的方式增强癌细胞的杀伤作用。为此,我们表明,膳食化合物马拉巴里酮C(mal C)产生了大量的活性氧(ROS),并且还降低了肺癌细胞中的GSH水平。矛盾的是,尽管补充抗氧化剂减少了C诱导的ROS的异常释放,硫醇抗氧化剂(NAC / GSH)恢复了细胞内GSH的水平,但增强了由mal C诱导的DNA DSB和凋亡细胞死亡。我们的结果揭示了归因于硫醇抗氧化剂这一敏化过程的两种紧密耦合的生化机制。首先,硫醇抗氧化剂可以使mal C发生“儿茶酚-醌氧化还原循环”,并改善ROS的产生和生物分子损伤(DNA和蛋白质)。其次,硫醇类抗氧化剂引起被mal C氧化的转录因子[p53,p65(NF-κB)等]快速谷胱甘肽酰化,并消除了它们的核螯合和抗凋亡基因的转录。此外,对p53表达和沉默细胞的线粒体组分的分析表明,谷胱甘肽化p53(p53-SSG)的细胞质积累触发了强大的线粒体死亡过程。有趣的是 p53中124、141和182位氧化还原敏感半胱氨酸残基的突变显着降低了mal C加NAC介导的癌细胞敏感性。在小鼠的两种不同肿瘤模型中的临床前结果进一步支持了我们的结论,即NAC能够敏化mal C诱导的体内肿瘤生长抑制作用。
更新日期:2020-01-13
中文翻译:

硫醇抗氧化剂通过在体外和体内对氧化还原敏感的p53和NF-κB蛋白进行重新编程,使马拉巴利康C诱导的癌细胞死亡敏感。
通过将药物靶向癌细胞的氧化还原稳态,对“氧化还原癌症疗法”的特别关注正在迅速增长。最近的临床研究表明,N-乙酰半胱氨酸(NAC)治疗可显着降低代谢异质性并降低Ki67(一种增殖标志物),并同时增强患者肿瘤细胞的凋亡。然而,尚不确切知道硫醇抗氧化剂如何以上下文相关的方式增强癌细胞的杀伤作用。为此,我们表明,膳食化合物马拉巴里酮C(mal C)产生了大量的活性氧(ROS),并且还降低了肺癌细胞中的GSH水平。矛盾的是,尽管补充抗氧化剂减少了C诱导的ROS的异常释放,硫醇抗氧化剂(NAC / GSH)恢复了细胞内GSH的水平,但增强了由mal C诱导的DNA DSB和凋亡细胞死亡。我们的结果揭示了归因于硫醇抗氧化剂这一敏化过程的两种紧密耦合的生化机制。首先,硫醇抗氧化剂可以使mal C发生“儿茶酚-醌氧化还原循环”,并改善ROS的产生和生物分子损伤(DNA和蛋白质)。其次,硫醇类抗氧化剂引起被mal C氧化的转录因子[p53,p65(NF-κB)等]快速谷胱甘肽酰化,并消除了它们的核螯合和抗凋亡基因的转录。此外,对p53表达和沉默细胞的线粒体组分的分析表明,谷胱甘肽化p53(p53-SSG)的细胞质积累触发了强大的线粒体死亡过程。有趣的是 p53中124、141和182位氧化还原敏感半胱氨酸残基的突变显着降低了mal C加NAC介导的癌细胞敏感性。在小鼠的两种不同肿瘤模型中的临床前结果进一步支持了我们的结论,即NAC能够敏化mal C诱导的体内肿瘤生长抑制作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号